Pharmaceuticals
Nippon Express (China) Opens New Healthcare Branch in Shanghai
- Company Is Stepping up Its Efforts in China's Pharmaceutical Industry - TOKYO, Aug. 1, 2022 /PRNewswire/ -- Nippon Express (China) Co., Ltd. (hereafter "NX China"), a group company of NIPPON EXPRESS HOLDINGS, INC., has established a Healthcare Branch inShanghai to strengthen its efforts in Chin...
HanAll Biopharma Reports Second Quarter 2022 Results and Provides Business Update
* Financial performance for Q2 2022 records revenues of KRW 26.2 billion, a 12% growth from the same period last year, driven by strong sales performance from the pharmaceutical division * Operating income turned to profit compared to the last quarter, while investment to R&D continued in t...
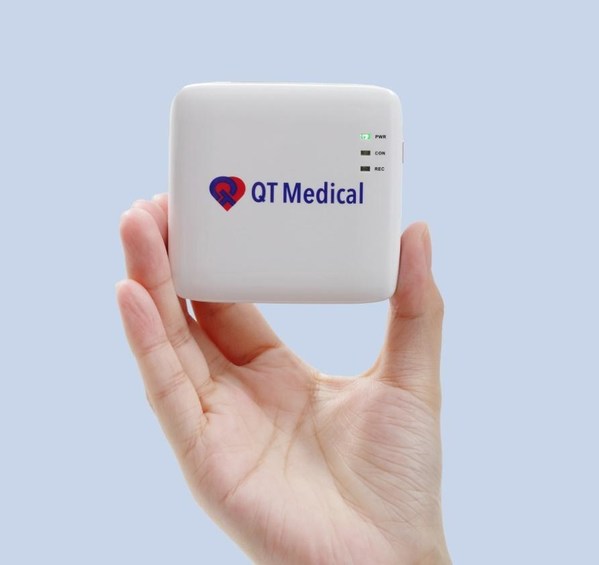
QT MEDICAL'S Online Order, Mail Delivery, XPRESS ECG Testing Service, to Exhibit at the World's Largest Annual Trade Show
Enabling patients to conduct electrocardiogram (ECG) tests safely and efficiently at home using PCA 500, the only medical grade 12-lead Resting ECG approved by the FDA for patient use. LAS VEGAS, July 29, 2022 /PRNewswire/ -- Demand for telehealth and home care has surged drastically during the ...
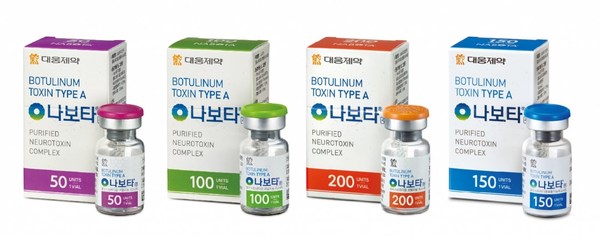
Daewoong Pharmaceutical Co., Ltd. revs up the growth engine for Nabota, achieving the highest quarterly performance ever
- Daewoong Pharmaceutical Co., Ltd. achieved 293.8 billion won in sales and 33.6 billion won in operating revenue in the second quarter due to the growth of ethical drugs and Nabota. - A continuous increase in profitability is expected due to sales visualization of the new drug Fexuclue and Na...
The Clinician and Clalit Health Services partner to drive value-based care and digital care pathway implementation in Israel
AUCKLAND, New Zealand and TEL AVIV, Israel, July 28, 2022 /PRNewswire/ -- The
Clinician
Plant-Oil-Based Skincare Technology Business Available for License or Purchase
A NEW AND UNIQUE ANHYDROUS SKINCARE TECHNOLOGY OF PROVEN VERY HIGH EFFICACY AUCKLAND, New Zealand, July 27, 2022 /PRNewswire/ -- Invented and developed by the CEO ofFutecNZ Limited in New Zealand, (trading under the ONE24 brand) trialled and tested by accredited scientific laboratories, a vast t...
Samsung Biologics Reports Second Quarter 2022 Financial Results
* Released consolidated financial position of the company based on its full acquisition of Samsung Bioepis completed onApril 20, 2022 * Achieved record-high semi-annual revenue exceeding KRW 1 trillion * Recorded Q2'22 revenue of KRW 503.7 billion for the company's CDMO business * Recorded ...
Nuance Pharma announces the completion of dosing for all NTM-001 Phase 1 clinical study participants in HKU Clinical Trials Centre
* All 16 healthy normal volunteers (HNVs) were dosed and no serious adverse event was reported * The NTM-001 Phase I clinical study is expected to be completed by the end of 2022 SHANGHAI, July 26, 2022 /PRNewswire/ -- Nuance Pharma ("the Company") announces the completion of dosing for all H...
Ascentage Pharma and Tanner Pharma Group Initiate a Global Innovative Named Patient Program
SUZHOU, China and ROCKVILLE, Md. and CHARLOTTE, N.C., July 25, 2022
/PRNewswire/ --Ascentage Pharma
Anti-PD-1 monoclonal antibody Puyouheng™ (HX-008, Pucotenlimab injection) Co-developed by LEPU BIOPHARMA and HANX BIOPHARMACEUTICALS was approved for marketing in China by NMPA
HANGZHOU, China, July 25, 2022 /PRNewswire/ -- Anti-PD-1 monoclonal antibody – PuyouhengTM (HX-008, pucotenlimab injection), was conditionally approved by the National Medical Products Administration (NMPA) for marketing inChina to treat patients with unresectable or metastatic microsatellite ins...
Ascentage Pharma Announces Clinical Trial Application for Olverembatinib (HQP1351) Approved in Canada
SUZHOU, China and ROCKVILLE, MD, July 21, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the Phase Ib study of Ascentage Pharma's novel...
ImmVira's intravenous administered oHSV MVR-T3011 IV demonstrated promising clinical biological activities
SHENZHEN, China, July 20, 2022 /PRNewswire/ -- ImmVira's global first intravenous ("IV") administered oncolytic herpes simplex virus ("oHSV") product MVR-T3011 IV has completed the first three cohort escalation in the U.S. Phase I clinical trial, tested dosage from 1x106 to 1x108 PFU on 10 subjec...
Daewoong Pharmaceutical Gets First Korean US FDA Fast-Track for New Idiopathic Pulmonary Fibrosis Drug
* US Food and Drug Administration (FDA) fast-tracks idiopathic pulmonary fibrosis treatment DWN12088 * First-in-class new drug to quickly take on the pulmonary fibrosis market, predicted to reach$6.1 billion globally by 2030 SEOUL, South Korea, July 20, 2022 /PRNewswire/ -- As many Korean p...
Ascentage Pharma and Innovent Announce the China NMPA Accepted and Granted Priority Review Designation to a New Drug Application for Olverembatinib for the Treatment of Drug-Resistant CML
SUZHOU, China, ROCKVILLE, Md. and SAN FRANCISCO, July 19, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, and Innovent Biologics, Inc. ("Innovent") (HKEX: 01...
Gracell Biotechnologies Appoints Veteran BioPharma Executive Dr. Samuel Zhang as Chief Business Officer
SAN DIEGO, Calif. and SUZHOU and SHANGHAI, China, July 19, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. ("Gracell" or the "Company", NASDAQ: GRCL), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of ...
OliPass Moves Forward to Second Stage of Phase 2a Trial for Pain Killer OLP-1002
SEOUL, South Korea, July 18, 2022 /PRNewswire/ -- OliPass Corporation, a South Korea based biotech specialized in the development of RNA therapeutics, announced to move forward to the second stage of the Phase 2a trial inAustralia in osteoarthritis (OA) patients for pain killer OLP-1002, an SCN9A...
Samsung Biologics to purchase land for its second Bio Campus
* Acquires additional land, sized 357,366㎡ valued at KRW 426 billion, for Bio Campus 2 * Plans to build Multi-Modal Plant, Open Innovation, and expanded manufacturing capacities INCHEON, South Korea, July 18, 2022 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), the world's leading contrac...
Everest Medicines Announces European Commission Grants Approval of Kinpeygo® for Adults with Primary IgA Nephropathy to our Partner Calliditas Therapeutics
Kinpeygo® (developed under the name NEFECON) is the first and only EMA- approved treatment for IgAN Everest Medicines has exclusive rights to develop and commercialize NEFECON in Greater China, Singapore and South Korea SHANGHAI, July 17, 2022 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Ev...
China CDE Grants Breakthrough Therapy Designation (BTD) to Neurophth's NR082 in LHON
WUHAN, China and SAN DIEGO, July 15, 2022 /PRNewswire/ -- Neurophth Therapeutics, Inc., ("Neurophth") today announced thatthe Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) has granted a Breakthrough Therapy Designation (BTD) to the Company's leading gen...
Bridge Biotherapeutics to Present Interim Clinical Data from Ongoing Phase 1 Study of BBT-176 at the World Conference of Lung Cancer
* The abstract suggests that BBT-176 has shown its capability of inducing clinical tumor responses in EGFR triple mutation-containing patients * Further exploration of the drug efficacy and safety profiles at RP2D is planned * The abstract is now available on the IASLC 2022 WCLC website SEON...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 328 media titles]
2026-02-24 12:00HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 309 media titles]
2026-02-26 09:30Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 307 media titles]
2026-02-26 19:57Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 298 media titles]
2026-03-02 09:15