Pharmaceuticals
Vernalis Research, a fully owned subsidiary of HitGen Inc., and Unison Medicines Inc. announce a research collaboration in the field of anti-infectives
CHENGDU, China, Sept. 5, 2022 /PRNewswire/ -- Vernalis Research ("Vernalis"), a fully owned subsidiary of HitGen Inc., and Unison Medicines Inc. ("Unison") are pleased to announce a research collaboration on an undisclosed bacterial target. Under the terms of the agreement, Vernalis will use it...
Simcere Pharmaceutical Announces Financial Results for 2022 H1: 27% Year-Over-Year Revenue Growth, with Innovative Drugs Accounting for 65.4% of Total Revenue
NANJING, China, Sept. 5, 2022 /PRNewswire/ -- Simcere Pharmaceutical Group Limited (2096.HK) announced its financial results for the first half of 2022. As ofJune 30, Simcere recorded operating revenue of RMB 2.7 billion for the first half of the year, with a year-over-year growth of 27.3%. The e...
Tamas Oravecz Ph.D. named SVP, CSO of the U.S. of Simcere Pharmaceutical Group
NANJING, China, Sept. 5, 2022 /PRNewswire/ -- Simcere Pharmaceutical Group announces today the appointment ofTamas Oravecz, Ph.D. as Senior Vice President, Chief Scientific Officer of the Simcere U.S. Tamas will be responsible for providing strategic leadership to our drug discovery efforts in A...
Jacobio Completes First Patient Dosage of CD73 mAb JAB-BX102 in China
BEIJING, Sept. 2, 2022 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced it has completed first patient dosage of it's in-house R&D drug candidate CD73 monoclonal antibody JAB-BX102 in a Phase I/IIa clinical trial for advanced solid tumour patients. This is a phase I/IIa multi-center, open-la...
Bridge Biotherapeutics Presented Non-clinical Study Results for 2 IPF Candidates at the IPF Summit 2022
* Non-clinical studies explored the potent anti-fibrotic and anti-inflammatory efficacy of BBT-301 and BBT-209 for IPF treatment * Company enhances its strategic focus on fibrotic diseases inclusive of IPF with 1 clinical asset and 2 non-clinical assets BOSTON and SEONGNAM, South Korea ,Sept. ...
Zhongchao Inc. Announces its New Strategy Extension Focusing on the Oncology and Other Major Disease Management
SHANGHAI, Sept 1, 2022 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), an internet technology company offering healthcare professionals the online healthcare information, professional training and educational services platform and patient management service, today a...
Licensing and Agency Agreement Signed between Beijing Minhai (Biokangtai) and Phil. Pharmawealth, Inc.
Marks the First Entry of Biokangtai's 13-valent Pneumococcal Vaccine into Southeast Asian Market SHENZHEN, China, Aug. 31, 2022 /PRNewswire/ -- On August 30, Beijing Minhai Biotechnology Co., Ltd. held a signing ceremony with Phil. Pharmawealth, Inc. for their cooperation in jointly promoting th...
GC Biopharma and Speragen Sponsored an Externally Led - Patient Focused Drug Development with FDA Attendance
YONGIN, South Korea and AUSTIN, Texas, Aug. 30 2022 /PRNewswire/ -- GC Biopharma (006280.KS) and Speragen today announced that a joint Externally Led - Patient-Focused Drug Development (EL-PFDD) meeting hosted by SSADH Association and attended by the U.S. FDA and other stakeholders was held to a...
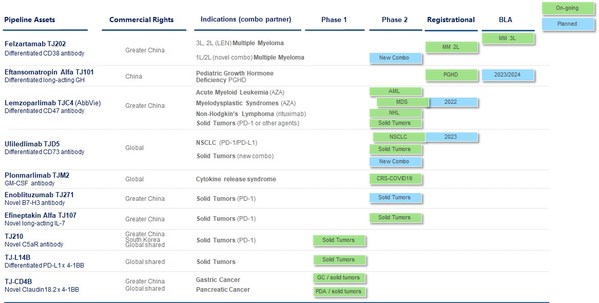
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Six Months Ended June 30, 2022
* Seven key clinical milestones achieved year-to-date, including positive data readouts for lemzoparlimab, uliledlimab, and TJ-CD4B * Lemzoparlimab is on track for Phase 3 study for 1L MDS * Amendment to the global partnership with AbbVie for certain new CD47 antibodies currently in developm...
Kintor Announces 2022 Interim Results and Recent Business Update
SUZHOU, China, Aug. 29, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma" or "the Company", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced its 2022 interim results and released an update on i...
NEXT-GENERATION EGFR TKI H002 FROM REDCLOUD BIO COMPLETES FIRST DOSE IN NON-SMALL CELL LUNG CANCER
* H002 is a precision therapeutic candidate for the treatment of NSCLC, with broad spectrum and high selectivity against multiple EGFR mutations including those with EGFR C797S mutation. * RedCloud Bio has initiated H002 Phase I/IIa clinical studies in both China and US. SHANGHAI, Aug. 29, 20...
Porton Advanced and Royaltech Announce Strategic Collaboration to Accelerate the Development of Bacterial Drugs and mRNA Drugs
SUZHOU, China, Aug. 29, 2022 /PRNewswire/ -- On August 19, 2022, Porton Advanced Solutions. (hereinafter referred to as Porton Advanced) announced a strategic cooperation with Suzhou Royaltech Med Co., Ltd. (hereinafter referred to as Suzhou Royaltech).This strategic collaboration will further in...
Sanyou Biopharmaceuticals and KangaBio established strategic collaboration to accelerate antibody drug development and innovation
SHANGHAI, Aug. 27, 2022 /PRNewswire/ -- Recently, Sanyou Biopharmaceuticals ( Shanghai) Co., Ltd. (Sanyou) and Shanghai KangaBio Co., Ltd. (KangaBio) have reached an antibody drug licensing agreement, regarding a proprietary monoclonal antibody drug developed by Sanyou, which grants KangaBio an e...
Ascentage Pharma Announces 2022 Interim Results
SUZHOU, China and ROCKVILLE, Md. , Aug. 26, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced its 2022 interim results. During the reporting p...
First approval of Cadonilimab (PD-1/CTLA-4 bispecific) published in Drugs, a peer-reviewed medical journal
HONG KONG, Aug. 26, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ("Akeso"), a biopharmaceutical company committed to the research, development, manufacturing and commercialization of either first-in-class or best-in-class therapies, announced a review article featuring onCadonilimab, a first-in-cla...
Alterity Therapeutics Launches ATH434 Phase 2 Clinical Trial for the Treatment of Patients with Multiple System Atrophy in Europe
Second region now open for enrolment for rare, rapidly progressive, neurodegenerative disease MELBOURNE, Australia and SAN FRANCISCO, Aug. 25, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing diseas...
111, Inc. Announces Second Quarter 2022 Unaudited Financial Results
SHANGHAI, Aug. 25, 2022 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced its unaudited financial results for the second quar...
Alpha Biopharma Announces Completion of its EVEREST Phase II/III Clinical Study of Zorifertinib in Non-Small Cell Lung Cancer Patients with Central Nervous System Metastases
* Zorifertinib is a next generation EGFR-TKI with full Blood Brain Barrier penetration targeting EGFRm+ NSCLC patients with CNS metastases * Top-line data from the study are expected around the end of 2022 SHANGHAI, Aug. 24, 2022 /PRNewswire/ -- Alpha Biopharma, a developer of innovative drugs...
Asieris Pharmaceuticals (688176.SH) Issued 2022 Semi-Annual Report: Accelerated Global Clinical Development and Progressive Implementation of Its Integrated Diagnosis-Treatment Commercialization Strategy
SHANGHAI, Aug. 24, 2022 /PRNewswire/ -- Asieris Pharmaceuticals, a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, issued its 2022 semi-annual report today. As disclosed in...
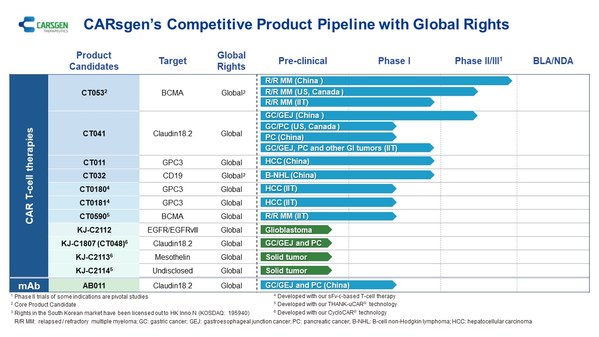
CARsgen 2022 Interim Results: Innovative CAR T-cell Technologies and Robust Pipeline
SHANGHAI, Aug. 24, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, has announced the 2022 Interim Results. Business Highlights * CT053: comple...
Week's Top Stories
Most Reposted
Visa partners with Laufey to spread the magic of travel in Asia Pacific; to be Official Payment Partner for Laufey: A Matter of Time Tour
[Picked up by 309 media titles]
2026-03-04 12:35SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15Infobip is set to launch AgentOS to orchestrate autonomous AI-driven customer journeys at scale
[Picked up by 290 media titles]
2026-03-02 09:00Klook's Spring Readiness Index shows how Asia's travelers are preparing for spring travel across Japan, South Korea, and Mainland China
[Picked up by 290 media titles]
2026-03-03 15:49COL and NASDAQ-Listed BeLive Holdings Unveil World's First "Microdrama in a Box" in Headline Hong Kong FILMART 2026 Launch
[Picked up by 284 media titles]
2026-03-05 17:14