Pharmaceuticals
Inmagene files two international patent applications
SHANGHAI and SAN DIEGO and HANGZHOU, China, Nov. 17, 2021 /PRNewswire/ -- Inmagene Biopharmaceuticals ("Inmagene" or the "Company") announced that it has filed two international patent applications for its drug candidates, IMG-007 (OX40 antagonist) and IMG-008 (IL-36R antagonist). One application...
Terumo Blood and Cell Technologies and Immunicom Establish Agreement to Launch Breakthrough Cancer Immunotherapy Treatment in Europe
* The therapy aims to re-energize a patient's immune system to fight cancer
tumors
* Initial commercialization will target leading oncology centers in Germany,
France, Italy, and Spain LAKEWOOD, Colo. and SAN DIEGO, Nov. 16, 2021
/PRNewswire/ --Terumo Blood and Cell Technologies
Senhwa Biosciences Announces Abstract Accepted for Presentation at the 2022 ASCO Gastrointestinal Cancers Symposium
TAIPEI and SAN DIEGO, Nov. 15, 2021 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that an abstract highlighting clinical design for their lead drug candidate...
Qilian International Holding Group Limited Announces Initiation of Development of New Drug for Gastric Cancer Prevention
JIUQUAN, China, Nov. 15, 2021 /PRNewswire/ -- Qilian International Holding
Group Limited (Nasdaq: QLI
Kintor Pharma Announces Dosing of First Patient in Phase II Clinical Trial of KX-826 for the Treatment of Androgenic Alopecia Female Patients in China
SUZHOU, China, Nov. 12, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced that the company has dosed the first patient in its phase II clinical ...
InnoCare to Attend Upcoming Investor Conferences to Share Latest Company Development
BEIJING, Nov. 12, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a leading biotech company, announced today that the company executives will attend the upcoming investor conferences organized by Goldman Sachs and Morgan Stanley to share company's latest development. Details are as follows: ...
PNOC Study in Childhood Brain Cancer Enrols First Patient
SYDNEY, Nov. 11, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that PNOC022 (NCT05009992), a multi-drug phase II study in DIPG and diffuse midline gliomas, has been initiated at theUniversity of Calif...
Gmax's GMA106, second generation obesity/T2DM/NASH mAb gives first in human dose
HANGZHOU, China, Nov. 11, 2021 /PRNewswire/ -- Gmax Biopharm today announces that the first dose of GMA106 was given to human subjects in a phase 1 study to investigate the safety, pharmacokinetics, and pharmacodynamics of this drug in the treatment of obesity. The study is a single dose, placeab...
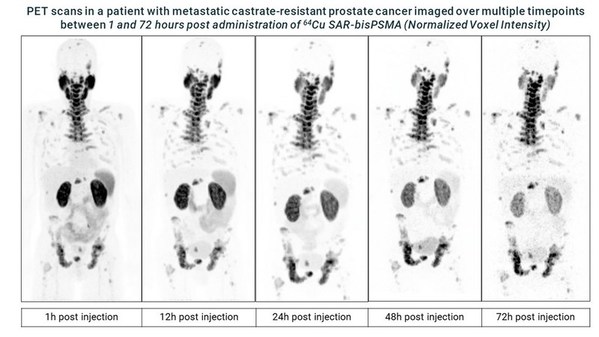
Recruitment for the dosimetry phase of Clarity's Cu-64/Cu-67 SAR-bisPSMA theranostic prostate cancer trial completed
* Clarity Pharmaceuticals completes recruitment for the initial dosimetry phase of its SAR-bisPSMA theranostic clinical trial SECuRE (NCT04868604)[1] investigating Targeted Copper Theranostics (TCT) in patients with metastatic castrate-resistant prostate cancer (mCRPC). * Dosimetry data is be...
IASO Biotherapeutics and Innovent Biologics to Present New BCMA CAR-T Cell Therapy Data in Oral Presentation at ASH 2021
SAN JOSE, Calif., NANJING, China and SHANGHAI, Nov. 10, 2021 /PRNewswire/ --
IASO Biotherapeutics("IASO Bio")
I-Mab and Jumpcan Announce Strategic Commercial Partnership on Eftansomatropin Alfa
* The partnership brings together the strengths of an innovative global biotech and a domestic leading pharmaceutical company specialized in and committed to pediatric medicines to accelerate the commercialization of eftansomatropin alfa * Jumpcan will pay I-Mab for a total of up to RMB 2.016...
Hummingbird Bioscience Announces Trials in Progress Poster Presentation at the Society for Immunotherapy of Cancer 2021 36th Annual Meeting
HOUSTON, Nov. 10, 2021 /PRNewswire/ -- Hummingbird Bioscience, an innovative clinical-stage biotech company focused on developing precision therapies against hard-to-drug targets, today announced a Trials in Progress poster presentation outlining the Phase 1 clinical trial design for HMBD-002, a ...
I-Mab and ABL Bio Report Preclinical Data of 4-1BB-targeting Bispecific Antibodies at 2021 SITC
* Preclinical data of TJ-CD4B/ABL111 and TJ-L14B/ABL503 demonstrate targeted safety profile and enhanced anti-tumor activity * Both studies are undergoing phase 1 clinical trials in the United States SHANGHAI and GAITHERSBURG, Md. and SEONGNAM, South Korea, Nov. 9, 2021 /PRNewswire/ -- I-Mab ...
Alterity Therapeutics Announces Presentation of ATH434 at the American Autonomic Society Virtual Meeting 2021
MELBOURNE, Australia, Nov. 9, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative conditions, today announced a poster presentation and accompanying vi...
FDA Clears Pharmaxis Cancer Drug to Progress to Phase 2 Study in Liver Cancer
SYDNEY, Nov. 9, 2021 /PRNewswire/ -- Clinical stage drug development company Pharmaxis Ltd (ASX: PXS) today announced that an Investigational New Drug application (IND) for a trial of PXS-5505 in hepatocellular carcinoma (HCC) patients has been cleared by the United States Food and Drug Administr...
Standigm Established a Synthetic Research Center to Improve Efficiency of AI Drug Discovery
SEOUL, South Korea, Nov. 8, 2021 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, announced today that the company had established a Synthetic Research Center in the headquarters of SK Chemicals Co., Ltd ("SK Chemicals", KRX 285...
Ascentage Pharma Announces Clinical Trial Agreement to Evaluate the Combination of Lisaftoclax (APG-2575) and the CDK4/6 Inhibitor IBRANCE® (Palbociclib) in Metastatic ER+/HER2- Breast Cancer
SUZHOU, China, and ROCKVILLE, Md., Nov. 8, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced a clinical trial collaborati...
I-Mab and Roche Diagnostics Announce Strategic Collaboration to Co-Develop Companion Diagnostics Solutions for I-Mab's Innovative Pipeline at the 4th CIIE
SHANGHAI and GAITHERSBURG, Md., Nov. 8, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the Company has entered into a strategic collaborati...
Everest Medicines Announces Up to HK$100 million Additional Share Repurchase Program
SHANGHAI, Nov. 7, 2021 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest" or the "Company"), a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products that address critical unmet medical needs for patients inAsia, today announced that i...
ASH 2021 | Ascentage Pharma to Release Latest Data from Two Studies of Its Bcl-2-Selective Inhibitor Lisaftoclax (APG-2575), Including a Chinese Study Demonstrating Complete Response
SUZHOU, China, and ROCKVILLE, MD., Nov. 4, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that abstracts on six studies of the company's thr...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 326 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 301 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09