Pharmaceuticals
MicuRx Pharmaceuticals Announces Promising Results from MRX-5 Study for Treating Mycobacterium abscessus Infections
FOSTER CITY, Calif., Oct. 8, 2024 /PRNewswire/ -- MicuRx Pharmaceuticals is excited to announce the publication of groundbreaking research on MRX-5, a novel oral oxaborole prodrug, demonstrating significant potential in the treatment of pulmonary infections caused byMycobacterium abscessus (Mab),...
Sciwind Biosciences Announces Oral Presentation on Oral GLP-2 Research Progress at the 32nd United European Gastroenterology Week (UEG Week 2024)
BEIJING and HANGZHOU, China, Oct. 7, 2024 /PRNewswire/ -- The 32nd United European Gastroenterology Week (UEG Week 2024) will take place inVienna, Austria , fromOctober 12 to 15, 2024. UEG Week is the largest gastroenterology conference inEurope and one of the leading meetings worldwide. It brings...
MediLink Announces Global Clinical Trial Collaboration and Supply Agreement on YL201 Combination Therapy
SUZHOU, China, Oct. 7, 2024 /PRNewswire/ -- MediLink Therapeutics (Suzhou) Co., Ltd. ("MediLink"), a clinical-stage biotech company, today announced a global clinical trial collaboration and supply agreement with Amgen Inc. Amgen will lead a global clinical study to evaluate the therapeutic poten...
TiumBio Announces First Patient Dosed in Phase 2 Clinical Trial of Oral Immuno-Oncology Drug TU2218
* TU2218 is a potentially first-in-class dual inhibitor targeting transforming growth factor beta receptor 1 (TGFR1) and vascular endothelial growth factor receptor 2 (VEGFR2) * The first patient with head and neck squamous cell carcinoma was dosed in the Phase 2a clinical trial of TU2218 *...
WELLlife COVID-19/Influenza A&B Home Test on Sale for Amazon Prime Day
Standfirst: Welllife, a Flu home test in addition to COVID-19 BOLINGBROOK, Ill., Oct. 3, 2024 /PRNewswire/ -- WELLlife, a leading innovator in medical diagnostic and testing solutions, is increasing accessibility to respiratory illness testing this flu season. The WELLlife COVID-19/Influenza A&B...
Doer Biologics Announces First Patient Dosed in Phase 2 Study of DR10624 for Treatment of Severe Hypertriglyceridemia
HANGZHOU, China, Sept. 30, 2024 /PRNewswire/ -- Zhejiang Doer Biologics Co., Ltd. ("Doer Bio"), a clinical stage biopharmaceutical company developing innovative biotherapeutics for metabolic diseases and cancers, today announces that DR10624, its first-in-class (FIC), tri-specific agonist targeti...
IASO Bio Scientist and Leading Chinese Physicians in Onco-Hematology Visit Hematology Center at the State University of Campinas, Brazil, to Explore Cooperation in Cancer Treatment
SHANGHAI and NANJING, China and SAN JOSE, Calif., Sept. 30, 2024 /PRNewswire/ -- OnSeptember 23, in Rio de Janeiro, on the eve of the International Myeloma Society (IMS) Annual Meeting, Dr.Yongke Zhang, Chief Scientific Officer of IASO Biotherapeutics (IASO Bio), along with leading Chinese onco-h...
Akeso's Cadonilimab Receives Second Indication Approval from NMPA for First-Line Treatment of Gastric/GEJ Cancer in All-Comers Population
HONG KONG, Sept. 30, 2024 /PRNewswire/ -- September 30, 2024, Akeso (9926. HK) announced that its internally developed PD-1/CTLA-4 bispecific antibody, cadonilimab, has received approval from the National Medical Products Administration (NMPA) for a new indication: cadonilimab in combination with...
Regor Enters into a Definitive Purchase Agreement for Genentech to Acquire Regor's Portfolio of next-generation CDK inhibitors for the Treatment of Breast Cancer
* Genentech will acquire Regor's next-generation CDK inhibitors for an upfront payment of$850 million in cash. Regor is also eligible to receive additional cash payments contingent on achieving future development, regulatory and commercial milestones CAMBRIDGE, Mass., Sept. 30, 2024 /PRNewswire...
SeromYx Systems and ACROBiosystems Announce Strategic Collaboration on Comprehensive Functional Profiling of Anti-CD20 Monoclonal Antibodies
NEWARK, Del., Sept. 29, 2024 /PRNewswire/ -- SeromYx Systems, a cutting-edge immunology technology company, and ACROBiosystems, an innovative provider for life science solutions and tools, are excited to announce the release of their joint study on the comprehensive functional profiling of approv...
IASO Bio Presented Comparative Clinical Outcomes on the Optimal Lymphodepletion Prior to Infution of Equecabtagene Autoleucel(FucasoTM)in Patients with Relapsed Refractory Multiple Myeloma at 2024 IMS Annual Meeting
SHANGHAI and NANJING, China and SAN JOSE, Calif, Sept. 29, 2024 /PRNewswire/ -- IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, today announced a poster presentati...
New Beginnings, New Journey | Sanyou Opening of New 10,000-sqm Facility, Ushering in a New Era of Innovation for Bio Drugs
SHANGHAI, Sept. 28, 2024 /PRNewswire/ -- Sanyou Bio recently celebrated a prestigious move into its new 10,000-square-meter R&D building atShanghai headquarters. This is not just a change in physical location, but a qualitative leap in the company infrastructure. It marks a solid step forward for...
111, Inc. Announces Receipt of Notification from Nasdaq
SHANGHAI, Sept. 27, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to reshaping the value chain of healthcare industry by digitally empowering the upstream and downstream inChina, today announced it has received a...
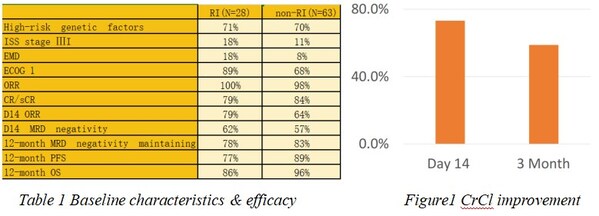
IASO Bio Presented the Outcomes of Relapsed/Refractory Multiple Myeloma (R/RMM) Patients with Renal Impairment Treated with Equecabtagene Autoleucel (Fucaso™) at 2024 IMS Annual Meeting
SHANGHAI and NANJING, China and SAN JOSE, Calif., Sept. 27, 2024 /PRNewswire/ -- IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, today announced a poster presentat...
Breakthrough Therapy Designation (BTD) for BioCity's SC0062, a selective endothelin type A (ETA) antagonist, granted by National Medical Products Administration for IgA nephropathy (IgAN) with proteinuria
SHANGHAI, Sept. 26, 2024 /PRNewswire/ -- BioCity Biopharma (BioCity) announced its endothelin receptor type A (ETA) selective antagonist SC0062 has been granted the Breakthrough Therapy Designation (BTD) by National Medical Products Administration (NMPA) for the treatment of IgA nephropathy (IgAN...
The World First Liquid Formulation of Recombinant Botulinum Toxin Type A Has Obtained IND Approval by the FDA
CHONGQING, China, Sept. 25, 2024 /PRNewswire/ -- MingMed Biotechnology, an innovative company focused on the in-house discovery and development of novel drugs, recently announced that its partially owned subsidiary, Claruvis Pharmaceutical Co., has received approval from the U.S. Food and Drug A...
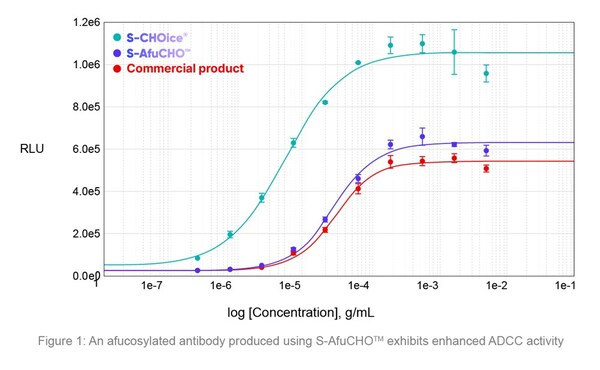
Samsung Biologics launches development platforms for enhanced therapeutic efficacy
* Samsung Biologics showcases new innovative development platforms – S-AfuCHO TM and S-OptiChargeTM – at BioProcess International 2024 * New technology platforms to proactively address evolving industry trends and enable high-quality development INCHEON, South Korea, Sept. 25, 2024 /PRNewswire...
XtalPi Launches Computational Chemistry Software for Drug Discovery: XMolGen and XFEP
BOSTON and SHANGHAI, Sept. 24, 2024 /PRNewswire/ -- Today, XtalPi announced the official launch of XFEP and XMolGen, two proprietary software products designed to accelerate and enhance the efficiency of drug discovery. Upon this launch, XtalPi will offer commercial licenses with flexible terms f...
LongBio announced the completion of Series B2 financing, led by Qiming Venture Partners
SHANGHAI, Sept. 24, 2024 /PRNewswire/ -- LongBio Pharma (Suzhou) Co., Ltd. ("LongBio" or the "Company"), a Phase III-stage biotech company focused on the R&D of innovative antibody and fusion protein drugs for allergies and complement-mediated diseases, announced the completion of its Series B2 ...
Nona Biosciences Enters Strategic Collaboration with Alkyon Therapeutics for Next-Generation Immunotherapeutics Discovery
CAMBRIDGE, Mass., Sept. 24, 2024 /PRNewswire/ -- Nona Biosciences, a global biotechnology company providing a total solution from "Idea to IND" (I to I™ ), announced today that it has entered into a strategic collaboration with Alkyon Therapeutics, Inc. (AlkyonTx), aSan Diego-based biotechnology ...
Week's Top Stories
Most Reposted
Edufrienz 99: One of Asia's First Digital Platform Advancing Character Education for Future-Ready, Compassionate Children
[Picked up by 317 media titles]
2026-01-23 11:03Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 308 media titles]
2026-01-27 13:00UOB prices AUD2 billion in five-year senior unsecured notes
[Picked up by 306 media titles]
2026-01-23 09:00AVPN's AI Opportunity Fund Expands Regional Efforts to Build AI Skilling Infrastructure for a Future-Ready Workforce Across Asia-Pacific
[Picked up by 299 media titles]
2026-01-21 12:39Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 297 media titles]
2026-01-22 15:16