Health
Lunit Announces Financial Results for the First Half of 2022
* Consolidated revenue in the second quarter of 2022 increased by 190% compared to same prior-year period * Total revenue for H1 2022 amounted to KRW 5.48 billion, or 82.5% of the total revenue of last year SEOUL, South Korea, Aug. 12, 2022 /PRNewswire/ -- Lunit (KRX: 328130.KQ), a global pro...
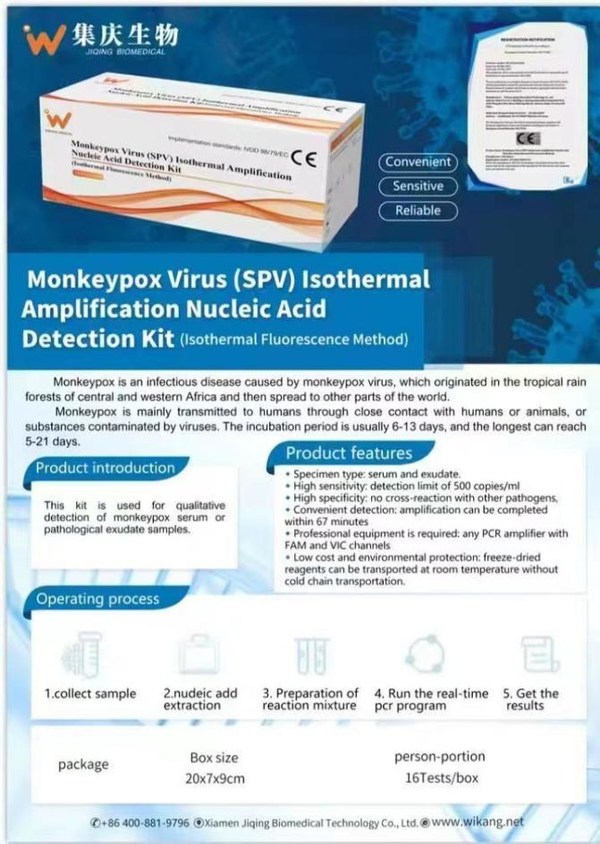
WeTrade Group Inc. Announces US$50 Million Sales of Monkeypox Virus Test Kits to Parkway Medical Limited
BEIJING, Aug. 12, 2022 /PRNewswire/ -- WeTrade Group Inc. ("Wetrade" or the "Company") (NASDAQ: WETG), an emerging growth company engaged in the business of providing software-as-a-services (SAAS) and cloud intelligent systems for micro-businesses, today announced the Company has entered into a s...
JIN-A02, A Novel 4th Generation EGFR TKI selected for the European Thoracic Oncology Platform - A First for Korean Pharma.
SEOUL, South Korea, Aug. 11, 2022 /PRNewswire/ -- J INTS BIO announced that the pre-clinical data of its novel, orally administered 4th generation EGFR TKI 'JIN-A02' was presented at the 2022 IASLC World Conference on Lung Cancer in Vienna, Austria on 8th August, at the official session "Overcomin...
WuXi ATU Announces Licensing Agreement with Janssen for TESSA™ Technology
PHILADELPHIA, Aug. 11, 2022 /PRNewswire/ -- WuXi Advanced Therapies (WuXi ATU), a wholly owned subsidiary of WuXi AppTec, today announced a licensing agreement with Janssen Biotech, Inc., one of the Janssen Pharmaceutical Companies of Johnson & Johnson ("Janssen"). Under this agreement, WuXi ATU ...
Zepp Health Corp. to Report Second Quarter 2022 Financial Results on August 25, 2022
Earnings Call Scheduled for 8:00 a.m. ET on August 25, 2022 BEIJING, Aug. 11, 2022 /PRNewswire/ -- Zepp Health Corp. ("Zepp Health" or the "Company") (NYSE: ZEPP), a cloud-based healthcare services provider with world-leading smart wearable technology, today announced that it will report its sec...
Panacell Biotech to conduct toxicity tests for NK cells, exosomes, and brown adipose-derived stem cells to treat patients with long COVID
SEOUL, South Korea, Aug. 10, 2022 /PRNewswire/ -- Panacell Biotech Co., Ltd.
said that NK cells, exosomes, and brown adipose-derived stem cells are
effective to treat patients with long COVID conditions, or post COVID-19
conditions, as well as those with terminal illness.
WeTrade Group Entered Into A Strategic Partnership With Jiqing, Acquiring Monkeypox Test Kits Exclusive Sales Channel
BEIJING, Aug. 10, 2022 /PRNewswire/ -- WeTrade Group Inc. ("WeTrade" or the "Company") (NASDAQ: WETG), an emerging growth company engaged in the business of providing software-as-a-services (SAAS) and cloud intelligent systems for micro-businesses, officially singed the strategic partnership agre...
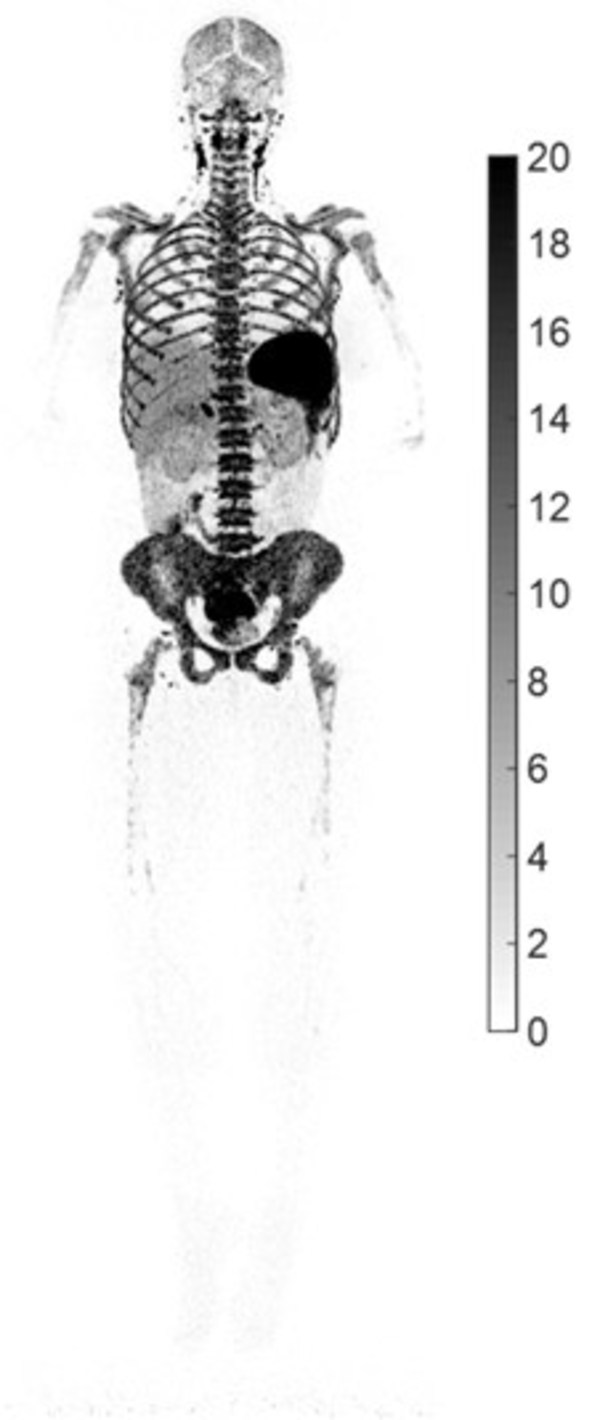
UC Davis' new research shows how total-body PET imaging can assess the immunological response to COVID-19 infections
HOUSTON, Aug. 10, 2022 /PRNewswire/ -- UC Davis, a leading university in molecular imaging that collaborated with United Imaging to develop the world's first total-body PET/CT scanner, 'uEXPLORER®,' recently presented their research on COVID-19 at the 2022 Society of Nuclear Medicine and Molecula...
Arbele Announces Phase 1 First-in-Human Study of CDH17xCD3 Bispecific T-Cell Engager for Treatment of Gastrointestinal Cancers
SEATTLE, Aug. 10, 2022 /PRNewswire/ -- Arbele, a clinical stage biopharmaceutical company, announces the successful dosing of the first patient inAustralia in Phase I Study of ARB202, for the treatment of advanced gastrointestinal cancers patients. Globally, Arbele is the first company exploring...
Asieris Appoints Dr. Badrinath Konety to Scientific Advisory Board
An expert in genitourinary oncology, Dr. Konety brings deep academic insight, broad clinical expertise and a global network to help Asieris advance its innovative pipeline to serve patients worldwide. SHANGHAI, Aug. 10, 2022 /PRNewswire/ -- Asieris Pharmaceuticals (stock code: 688176.SH), a glob...
Full-Life Technologies Deepens Radioisotope Technology Expertise with Key Leadership Appointments and the Establishment of a Radioisotope Technology Advisory Board
Co-founder and Board Member Hong-hoi Ting, D.Phil. Appointed Chief Strategy Officer James McCarter, Ph.D. Named Vice President, Head of Radioisotope Technology Research William Diamond, Ph.D., Carl Ross, Ph.D. and Herbert Moore, Ph.D. Join Radioisotope Technology Advisory Board SHANGHAI and BR...
Bridge Biotherapeutics Presents Promising Interim Clinical Study Results from Phase 1 Study of BBT-176 at WCLC 2022
* The study confirmed preliminary efficacy and safety of BBT-176 along with its treatment responses in advanced NSCLC patients * A patient with the C797S triple mutation showed up to 53 percent reduction in EGFR mutant allelic change VIENNA and SEONGNAM, South Korea, Aug. 9, 2022 /PRNewswire/...
iNtRON developed New CRISPR/Cas technology specific for Bacteriophage as PHAGERUS® Platform
* CRISPR/Cas system for bacteria that are not E. coli or Streptococcus pyogenes * Customized gene editing technology for unique modified-bacteriophages * To validate efficacy of SARS-CoV-2 mimotopes loaded PHAGERUS® platform BOSTON and SEOUL, South Korea, Aug. 9, 2022 /PRNewswire/ -- iNtRON ...
uAssist™ aligner planning assistance service available for orthodontists
MEMPHIS, Tenn., Aug. 10, 2022 /PRNewswire/ -- uLab Systems™, the creator of the uSmile™ clear aligner system and the uDesign® treatment planning software, announces the availability of the uAssist™ concierge treatment planning assistance service1 for orthodontists. Licensed orthodontists and dent...
Ok Away takes off with Air Doctor partnership
MELBOURNE, Australia, Aug. 9, 2022 /PRNewswire/ -- Global travel and insurtech start-up, Ok Away has announced a new partnership with tech Company - Air Doctor. Ok Away, a B2C mobile SaaS app, allows family and friends to connect better while apart through sharing mapped location, address, local...
Kintor Pharma Announces Completion of Subject Enrollment and Dosing in Phase I Clinical Trial of AR-PROTAC(GT20029) in China
SUZHOU, China, Aug. 9, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that the company has completed the enrollment and dosing of 92 subjec...
Harbour BioMed to Announce 2022 Interim Results on August 31, 2022
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 9, 2022 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142) announced that it will report financial results for the first half year endedJune 30, 2022, on Wednesday, August 31, 2022. The Company will host an English virtual ...
Inmagene and HUTCHMED Announce First Participants in Global Phase I Trial of IMG-004
SAN DIEGO, SHANGHAI and HONG KONG, Aug. 9, 2022 /PRNewswire/ -- Inmagene
Biopharmaceutical ("Inmagene
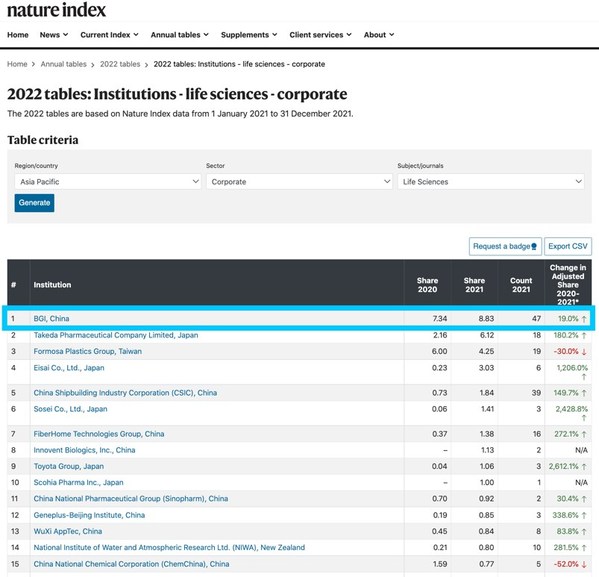
BGI Ranks No. 1 among APAC and China Life Science Corporations for Seven Consecutive Years: 2022 Nature Index Annual Tables Revealed
SHENZHEN, China, Aug. 8, 2022 /PRNewswire/ -- BGI has topped the Asia Pacific andChina life science corporate institution ranking table for the seventh year running, released in the 2022 Nature Index Annual Tables. With a 19 percent increase in its adjusted share metric, BGI ranked eighth among g...
WeTrade Group intends to enter exclusive strategic partnership with Jiqing Bio Regarding Exclusive Monkeypox Test Kits Sales Channel globally
BEIJING, Aug. 8, 2022 /PRNewswire/ -- WeTrade Group Inc. (NASDAQ: WETG), an emerging growth company engaged in the business of providing software-as-a-services (SAAS) and cloud intelligent systems for micro-businesses, completed an in-depth discussion of the strategic partnership with Jiqing Bio...
Week's Top Stories
Most Reposted
Brankas Launches Integrated APAC Open Banking Compliance Solution with ADVANCE.AI's eKYC Solution
[Picked up by 319 media titles]
2024-11-07 09:00Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 311 media titles]
2024-11-12 09:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
2024-11-11 21:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 288 media titles]
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 285 media titles]
2024-11-12 11:00