Health
WuXi XDC Successfully Listed on the Main Board of Hong Kong Stock Exchange
HONG KONG, Nov. 17, 2023 /PRNewswire/ -- WuXi XDC Cayman Inc. ("WuXi XDC" or the "Company", stock code: 2268.HK), a leading global Contract Research, Development and Manufacturing Organization ("CRDMO") focused on antibody drug conjugates ("ADC") and the broader bioconjugate market, listed on the...
AIRS Medical and Strategic Radiology (SR) Finalize Partnership Agreement Ahead of RSNA's 2023 Annual Meeting
SEOUL, South Korea, Nov. 16, 2023 /PRNewswire/ -- AIRS Medical, the leading AI-powered healthcare solution provider, has finalized an enterprise-wise partnership with Strategic Radiology (SR), a rapidly growing member-owned coalition of 37 privately owned, independent local radiology practices c...
Once-Weekly TransCon™ CNP Achieved Primary Efficacy Objective in ACcomplisH China Phase 2 Trial
SHANGHAI, Nov. 16, 2023 /PRNewswire/ -- VISEN Pharmaceuticals (VISEN), an innovative biopharmaceutical company focused on endocrine diseases, is pleased to announce the topline results from the ACcomplisH China Phase 2 Trial in children with achondroplasia (ACH) aged 2 to 10 years. In the trial, ...
Complete Genomics opens $3.2 million manufacturing facility at its San Jose headquarters
SAN JOSE, Calif., Nov. 16, 2023 /PRNewswire/ -- Complete Genomics, a pioneering genomic sequencing company, today announced plans for the opening of a$3.2 million manufacturing facility within its headquarters in San Jose, California. The facility, scheduled to open inMarch 2024, will give Comple...
Lunit to Implement AI-Powered Chest Screening Solution in Five Major Saudi Hospitals
- Lunit brings Lunit INSIGHT CXR to five major Saudi hospitals, aiming to enhance early detection of common lung abnormalities and improve patient outcomes in line with the national health strategy SEOUL, South Korea, Nov. 16, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of ...
Heuron to Participate in RSNA 2023, Showcasing Neuro AI Solutions and Presenting Abstract on AI Solutions for Stroke
SEOUL, South Korea, Nov. 16, 2023 /PRNewswire/ -- Heuron, a medical AI (artificial intelligence) imaging software solution company, under the leadership of CEO Dr.Donghoon Shin, is scheduled to take part in the 2023 Radiological Society ofNorth America (RSNA) conference, scheduled for this month...
Holmusk's NeuroBlu Database Reaches Pivotal Milestone of 4 Million Patient Lives
The NeuroBlu Database is now the largest NLP-enriched, de-identified real-world data platform designed for behavioral and mental health NEW YORK, Nov. 16, 2023 /PRNewswire/ -- Holmusk, a leading global behavioral health real-world evidence company, today announced that NeuroBlu has reached a maj...
ACROBiosystems launches GMP-grade DLL4 Protein to accelerate stem cell manufacturing
NEWARK, Del., Nov. 16, 2023 /PRNewswire/ -- ACROBiosystems, a global cornerstone of the pharmaceutical industry committed to offering innovative tools and solutions, recently announces the launch of GMP grade DLL4. GMP grade DLL4 is a recombinant, soluble form of Delta-like Ligand 4, or DLL4, ...
Origin Agritech to Hold Business Update Conference Call on Tuesday, November 21 at 8 a.m. ET
BEIJING, Nov. 15, 2023 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), a leading Chinese agricultural technology company, will host a conference call onTuesday, November 21, at 8:00 AM ET (9:00 PM Beijing Time) with the investment community to discuss the Company'...
dsm-firmenich and Boncha Bio Establish Partnership to Advance Nutraceuticals with Candy-capsule Technology in Asia
TAIPEI, Nov. 15, 2023 /PRNewswire/ -- Reshape the Future of Nutritional Supplements In a landmark development, Boncha Bio is elated to announce a strategic partnership inAsia with dsm-firmenich, a global innovator in nutrition, health, and beauty. This groundbreaking partnership was ceremoniously...
Lunit Secures $150 Million in Capital Increase
* Fund fully paid-in as of November 9, achieving over 100% shareholder participation SEOUL, South Korea, Nov. 15, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, today announced the successful completion of a signi...
CCHIO, a Milestone Event for the Global Cancer Community, Returns to Tianjin in 2023
Bringing together expertise to pool wisdom in China and around the world. TIANJIN, China, Nov. 15, 2023 /PRNewswire/ -- The 2023 edition of the Chinese Congress of Holistic Integrative Oncology will be held fromNovember 16th to 19th in Tianjin,China. Organized by the China Anti-Cancer Association...
Riding the tide, realize the future: The BD paths for Chinese Biotech
WUXI, China, Nov. 14, 2023 /PRNewswire/ -- 2023 BIO-Europe just closed last week. In line with previous conferences, this BD carnival attracted more than 2,000 companies, 5,000 attendees from close to 60 countries all over the world. Recently, we interviewed a global BD expert, Dr. Kaan Certel. ...
2023 World Laureates Association Prize winners awarded in Shanghai
SHANGHAI, Nov. 14, 2023 /PRNewswire/ -- The five winners of the 2023 World Laureates Association Prize (WLA Prize) received their awards for their ground-breaking achievements in computer science or mathematics and life science or medicine onNov. 6 at the opening ceremony of the 6th WLA Forum hel...
EDAN's Focus on Medical Technology Advancement Shines at MEDICA 2023
DUSSELDORF, Germany, Nov. 14, 2023 /PRNewswire/ -- Edan Instruments Inc.
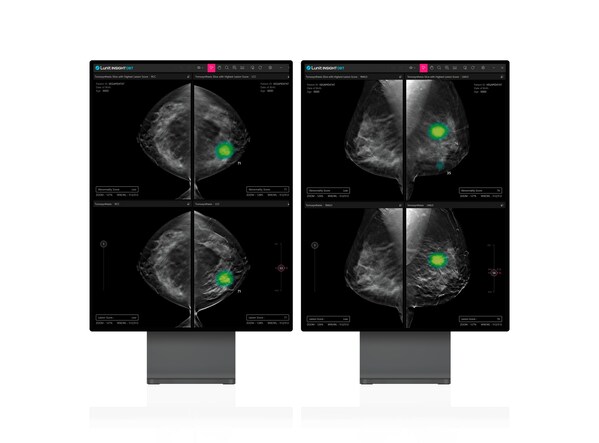
Lunit Nets FDA Nod for AI-Powered 3D Breast Tomosynthesis Solution, Lunit INSIGHT DBT
- Lunit INSIGHT DBT receives FDA 510(k) clearance, poised to enter the world's largest DBT market SEOUL, South Korea, Nov. 14, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, today announced that it has received U.S....
CytoMed Therapeutics to Present at The Benchmark Company's Upcoming Discovery One-on-One Investor Conference
SINGAPORE, Nov. 14, 2023 /PRNewswire/ -- CytoMed Therapeutics Limited
Wyeth Becomes the First Multinational Company to Launch an HMO Growing-Up Infant Formula in China
SUZHOU, China, Nov. 14, 2023 /PRNewswire/ -- On November 13th, 2023, Wyeth Nutrition announced the launch of its first illuma HMO Growing-up infant formula added with two human milk oligosaccharides (HMOs) inChina. Designed for babies above 3 years old, this product is manufactured at the company...
HaemaLogiX Announces Appointment of Damian Clarke-Bruce as Managing Director and CEO
SYDNEY, Nov. 13, 2023 /PRNewswire/ -- HaemaLogiX Ltd, a clinical stage Australian biotech, is pleased to announce the appointment ofDamian Clarke-Bruce as Managing Director and CEO, effective13 November 2023. Outgoing CEO Bryce Carmine will remain active in the company, retaining his role as Cha...
Brii Bio Presents New Data Highlighting Progress Towards Achieving HBV Functional Cure at AASLD's The Liver Meeting® 2023
Direct evidence that BRII-179-induced functional antibody responses can contribute to increased and sustained HBsAg loss rate New insight in utilizing BRII-179 to enriching patients with intrinsic humoral immune responses for higher HBsAg loss or HBV functional cure rates New data support ...
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 292 media titles]
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
2024-11-11 21:00DND International Eye Hospital: Pioneering SMILE Pro Surgery & Leading the Trend of Refractive Surgery Tourism in Vietnam
[Picked up by 279 media titles]
2024-11-08 20:11