Health
Zhongchao Inc. Announces Successful Medication Assistance Services for Over 7,000 SLE Patients with Approximately 130,000 Units of Medications
SHANGHAI, March 12, 2024 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), a platform-based internet technology company offering services for patients with cancer and other major diseases, today announced that Shanghai Zhongxin Medical Technology Co., Ltd. ("Zhongxin")...
GenScript Biotech Announces 2023 Annual Results with a Five-Year CAGR stands at 30%
R&D Innovation to Lead the Future, Deepening Customer Value Creation * Sustained High Revenue Growth: GenScript Group's five-year CAGR stands at an impressive 30%, with a strong global operation and stable cash flow. In 2023, the group's best-in-class cell therapy product CARVYKTI®, achieved sa...
Longbio Pharma Presented Positive Phase 1 Results for LP-003 at 2024AAD
SAN DIEGO, March 12, 2024 /PRNewswire/ -- Longbio Pharma (Suzhou) Co., Ltd. (referred to as "Longbio Pharma"), a leading biotech company dedicated to developing innovative protein treatments for allergy, respiratory, dermatology, hematology, ophthalmology, and other autoimmune and rare diseases, ...
Baird Medical and ExcelFin Acquisition Corp Announce Strategic Update to Business Combination Terms to Reinforce Long-Term Value Creation Opportunity and Alignment with Shareholders
* Baird Medical agreed to subject 30% of its shares to be received in the transaction to an earnout at a$12.50 trading price * Minimum cash condition of $15 million has been waived, cementing certainty and expediency to our transaction close * Upon closing, Baird Medical is expected to trade...
Everest Medicines' Partner Calliditas Therapeutics Announces U.S. FDA Grants an Additional Seven-Year Orphan Drug Exclusivity Period for Nefecon®
SHANGHAI, March 11, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company")'s licensing partner Calliditas Therapeutics AB (Nasdaq: CALT, Nasdaq Stockholm: CALTX) ("Calliditas") announced that the U.S. FDA has granted an orphan drug exclusivity period of seven years for...
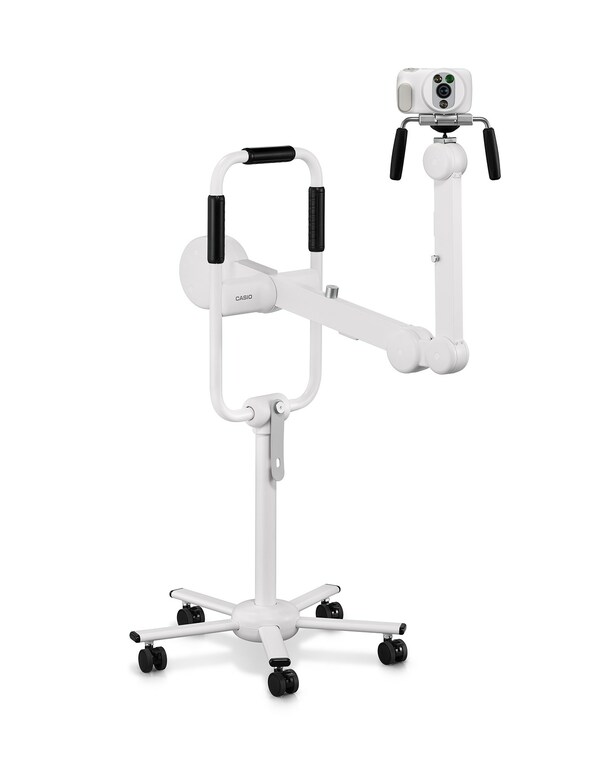
Casio to Release Uterocervical Observation Camera in the United States, Australia and New Zealand
FDA Registration Completed in the United States and TGA Approval Obtained in Australia TOKYO, March 11, 2024 /PRNewswire/ -- Casio Computer Co., Ltd. announced today that the DZ-C100 COLPOCAMERA, which is designed for uterocervical observation and photography in gynecological settings, and the C...
Denali Capital Acquisition Corp. Announces Extension of Deadline to Complete Business Combination
NEW YORK, March 11, 2024 /PRNewswire/ -- Denali Capital Acquisition Corp. (NASDAQ: DECA) (the "Company") announced today that an aggregate of$50,000 has been deposited into the Company's trust account to further extend the period of time the Company has to consummate its business combination by a...
Mabwell to Present ADC Platform IDDC™ and the Latest Study Results of Multiple Novel ADCs at the 14th World ADC London
SHANGHAI, March 11, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present its next generation ADC platform IDDC™ and the latest study results of multiple novel ADCs (9MW2821, 7MW3711, 9MW2921) developed...
Medicilon has appointed Dr. Qingcong Lin as President of Medicilon USA Corp., further deepening the global strategic layout
BOSTON, March 11, 2024 /PRNewswire/ -- Medicilon, a one-stop pharmaceutical preclinical research and developmentCRO can simultaneously meet Chinese and American drug discovery and development needs (including GLP standards) promptly and efficiently with fast initiation times.Over the past 20 year...
TiumBio to Initiate Phase 2 Trial of TU2218 for Cancer
* TiumBio will pursue three indications for the TU2218 combination clinical trial with pembrolizumab * TU2218 demonstrated a favorable safety and a synergetic effect with immuno-oncology drugs in preclinical studies BOSTON and SEONGNAM, South Korea, March 11, 2024 /PRNewswire/ -- TiumBio Co.,...
ONO Enters into a University-Wide, Research Alliance Agreement with Harvard University
OSAKA, Japan, March 11, 2024 /PRNewswire/ -- Ono Pharmaceutical Co., Ltd. ( Osaka, Japan; President and CEO: Gyo Sagara; "Ono") today announced that it entered into a five-year, university-wide strategic research alliance agreement withHarvard University (Cambridge, MA, USA; "Harvard") aiming at v...
AirNet Announces Changes in the Board of Directors
BEIJING, March 11, 2024 /PRNewswire/ -- AirNet Technology Inc., formerly known as AirMedia Group Inc. (the "Company") (Nasdaq: ANTE), today announced changes in its board of directors (the "Board"). Mr. Dong Wen has tendered resignation as an independent director of the Company, a member of the ...
Ascletis Announces Poster Presentation of Phase II Study Final Results of FASN Inhibitor ASC40 for Treatment of Acne at 2024 AAD Annual Meeting
HANGZHOU and SHAOXING, China, March 10, 2024 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces the poster presentation of Phase II study final results of ASC40, a first-in-class fatty acid synthase (FASN) inhibitor for treatment of acne, at the 2024 American Academy of...
Regor Initiates Phase 2 Study of Oral Once-daily GLP-1 Agonist RGT-075 for the Treatment of Obesity
CAMBRIDGE, Mass., March 8, 2024 /PRNewswire/ -- Regor Therapeutics Group ("Regor"), a clinical-stage global biopharmaceutical company powered by a cutting-edge drug discovery engine and differentiated clinical development pipeline, today announced that its Phase 2 trial of the highly selective or...
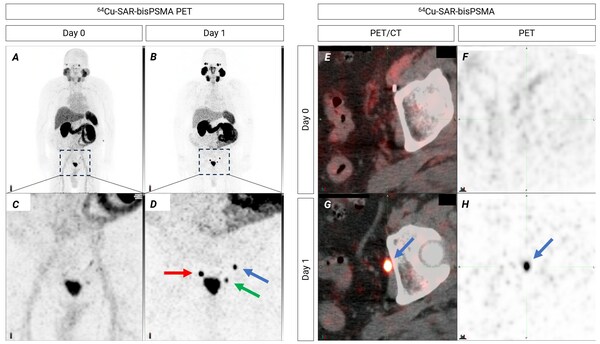
Additional COBRA results: SAR-bisPSMA detects lesions in the 2-millimetre range
Highlights * Clarity recently reported that in its diagnostic Phase 1/2 trial, COBRA, 64 Cu-SAR-bisPSMA was found to be safe and highly effective in detecting prostate cancer (PC) lesions in patients with biochemical recurrence (BCR). * In trial participants where standard of care (SOC) imagin...
Wellysis and Artella Solutions Launch Innovative Remote Cardiac Monitoring Service in the US.
SEOUL, South Korea, March 8, 2024 /PRNewswire/ -- Wellysis, a digital
healthcare company spun off from Samsung, has announced the launch of remote
cardiac monitoring service in the US in partnership with Artella Solutions
(ARTELLA).
Mabwell to Present the Clinical Data of 9MW2821 in Cervical Cancer as Focused Plenary Oral Presentation at 2024 Society of Gynecologic Oncology Annual Meeting on Women's Cancer
SHANGHAI, March 8, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present the clinical study results on the efficacy and safety of the novel Nectin-4-targeting ADC 9MW2821 for patients with recurrent or ...
TransThera Announced Global Phase 3 Clinical Trial for Cholangiocarcinoma Authorized in the European Union and Orphan Drug Designation for Tinengotinib to Treat Biliary Tract Cancer Granted by European Medicines Agency
NANJING, China and GAITHURSBURG, Md., March 8, 2024 /PRNewswire/ -- TransThera, a clinical-stage biopharmaceutical company dedicated to innovating differentiated drugs globally, today announced that the randomized, controlled, global multicenter Phase 3 trial (FIRST-308) of tinengotinib versus ph...
VISEN Announces Acceptance of a Biologics License Application for Lonapegsomatropin in China
SHANGHAI, March 7, 2024 /PRNewswire/ -- VISEN Pharmaceuticals (VISEN), an innovative biopharmaceutical company focused on endocrine diseases, today announced that the Biologics License Application (BLA) for Lonapegsomatropin (TransCon hGH) was accepted by the China National Medical Products Admi...
Advancing Healthcare Access: Genesis MedTech Teams Up with Silk Road Medical to Serve Patients affected by Carotid Artery Disease in China
WUXI, China, March 7, 2024 /PRNewswire/ -- Silk Road Medical (NASDAQ: SILK), a medical device company based inCalifornia USA and Genesis MedTech Group have signed an exclusive distribution agreement to introduce the TCAR® core products, ENROUTE® Transcarotid Neuroprotection System and ENROUTE® T...
Week's Top Stories
Most Reposted
Labuan IBFC Inc. and STEP Malaysia jointly host wealth management and estate planning event
[Picked up by 318 media titles]
2024-06-28 12:45Co-creating a Digital and Intelligent World, H3C Digital Tour 2024 Concludes Successfully
[Picked up by 293 media titles]
2024-07-04 12:02Yidu Tech Announces Annual Results for FY 2024: Existing Business Achieves First Full-Year Profit on Adjusted EBITDA
[Picked up by 291 media titles]
2024-07-01 09:05Hukumonline Launches Comprehensive Law Firm Directory As a Guide for Investment and Business in Indonesia
[Picked up by 285 media titles]
2024-06-28 21:46AI and Sustainability in Advanced Manufacturing to take Centre Stage at Industrial Transformation ASIA-PACIFIC (ITAP) 2024
[Picked up by 283 media titles]
2024-07-03 15:51