 |
Highlights
SYDNEY, March 8, 2024 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to share additional data from its diagnostic 64Cu-SAR-bisPSMA trial, COBRA (NCT05249127)[1].
COBRA was a multi-centre, single-arm, non-randomised, Phase 1/2 diagnostic imaging study of 64Cu-SAR-bisPSMA administered to participants with BCR of PC following definitive therapy and who had a negative or equivocal SOC scan at screening. The primary objectives of the trial were to investigate the safety and tolerability of 64Cu-SAR-bisPSMA as well as its ability to correctly detect recurrence of PC. Patients underwent PET/computed tomography (CT) scans with 64Cu-SAR-bisPSMA on Day 0 and Day 1 (1-4h and 24±6h post-dose, respectively), which were interpreted by three blinded central readers. Following the recent announcement of positive results from the COBRA trial[2], which began showcasing the many benefits of 64Cu-SAR-bisPSMA, further analysis of the data reveals additional advantages of this optimised PSMA product.
64Cu-SAR-bisPSMA was able to detect much smaller lesions than anticipated, including a lesion with a diameter of less than 2 mm. This compares favourably against the current SOC PSMA PET imaging agents, including PYLARIFY® and the generic product 68Ga-PSMA-11, with which the detection of lesions smaller than 5 mm is challenging. Sensitivity is a known challenge for the existing PSMA PET agents, particularly for lesions <5 mm[3]-[6]. This suggests that lesions are missed by current SOC imaging, which can have significant implications on accurate staging and subsequent treatment decisions. For those undergoing initial staging of their disease, missing lesions may lead to unnecessary surgery resulting in long-lasting side effects (e.g. impotence and/or incontinence following the removal of the prostate)[7]. In patients with BCR of PC, it is also crucial to identify their cancer early to avoid disease progression and the side effects of systemic treatments that accompany such therapies[8].
Clarity's Executive Chairperson, Dr Alan Taylor, commented, "The cornerstone of better therapy is better diagnosis, and we are incredibly excited about the substantial degree of improvement in detection of lesions with our bisPSMA product compared to SOC imaging. This difference is similar to comparing the old Hubble telescope to the new James Webb telescope, allowing us to visualise with much greater clarity, and to effectively change the paradigm of treatment to considerably improve the outcomes for patients with PC. Innovative products like 64Cu-SAR-bisPSMA may prevent many millions of men from receiving inappropriate treatments and suffering substantial debilitating side effects from surgery or other therapies.
"When combining the optimised dual PSMA targeting agent with the ideal half-life of copper-64 (64Cu), we continue to observe higher uptake and retention of 64Cu-SAR-bisPSMA in PC lesions. We believe the ability of 64Cu-SAR-bisPSMA to detect such small lesions is due to the higher uptake and retention of the product over time with high contrast (parameters measured by mean standardised uptake value [SUVmean], maximum standardised uptake value [SUVmax] and tumour-to-background ratio [TBR]). Small lesions become more readily detectable if the uptake of the product in the lesion is high and the background noise on the image is low. This effect enhances the contrast, making the lesion easier to detect, providing clinicians with valuable information on how to best treat their patients."
The size of the PC lesions detected by 64Cu-SAR-bisPSMA was recorded on the same-day (Day 0) and next-day (Day 1) imaging. Lesions with less than 5 mm in size were identified across readers among 14% (7/50) of patients (Figures 1 and 2). These lesions were located in the bone, pelvic and extra-pelvic lymph node regions. The smallest lesion (pelvic lymph node) identified in the study was 2.6 x 1.9 mm (Figure 3).
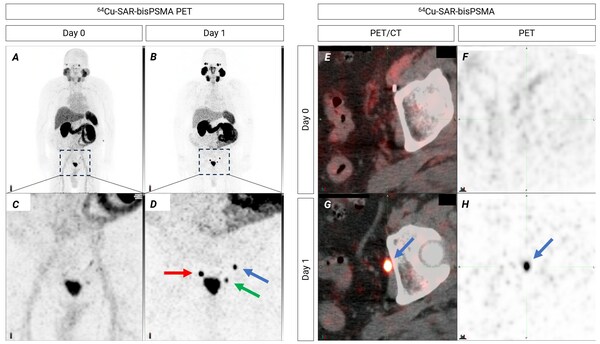
Figure 1. Pelvic lymph nodes showing uptake of 64Cu-SAR-bisPSMA on Day 1 (arrows). Blue arrow: lesion size 3.8mm x 4.4mm, SUVmean 20.6, SUVmax 22.1 and TBR 130.1. Green arrow: lesion size also 3.8mm x 4.4mm, SUVmean 11.9, SUVmax 12.8 and TBR 75.3. Red arrow: lymph node showing 64Cu-SAR-bisPSMA uptake (>5mm). A,B,C,D: Maximum Intensity Projection. Inset in top images displays pelvic region (bottom images). E,F,G,H: axial view of pelvic region showing the same lymph node as on image D (blue arrow)
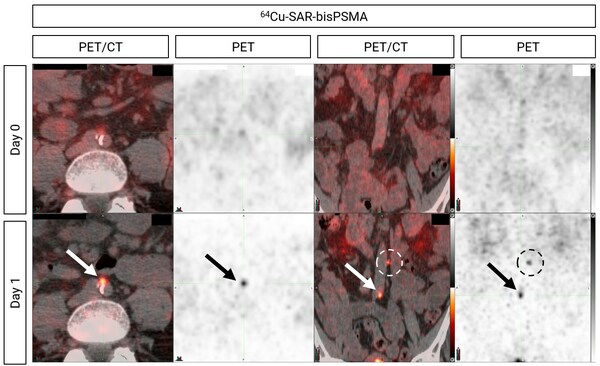
Figure 2. Extra-pelvic lymph nodes (retroperitoneal) showing uptake of 64Cu-SAR-bisPSMA on Day 1 (arrow and circle, bottom images). Arrow: lesion size 2.4mm x 3mm. SUVmean 8.3, SUVmax 8.5 and TBR 77.1. Circle: additional extra-pelvic lymph node showing uptake of 64Cu-SAR-bisPSMA. Extra-pelvic region confirmed by histopathology as positive for PC. Left images: axial view. Right images: coronal view.
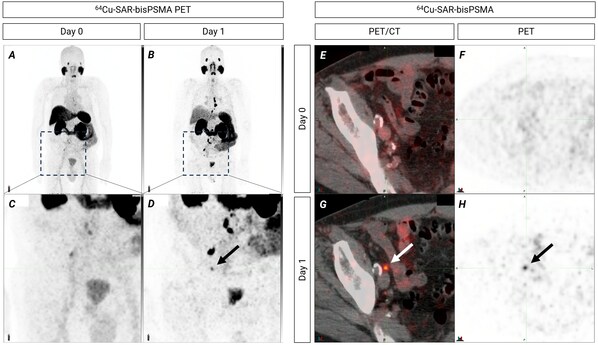
Figure 3. PET images showing lymph nodes with uptake of 64Cu-SAR-bisPSMA better visualised on Day 1 (pelvic and extra-pelvic regions – retroperitoneal and supradiaphragmatic). Right pelvic lymph node showing uptake of 64Cu-SAR-bisPSMA on Day 1. Lesion size: 1.9mm x 2.6mm. SUVmean 8.0, SUVmax 8.2 and TBR 67.9. A,B,C,D: Maximum Intensity Projection. Insert in top images displays pelvic and extra-pelvic regions. E,F,G,H: axial view of pelvic region showing the same lymph node as on image D (arrow).
The SUVmax, SUVmean and TBR were assessed in up to 25 lesions per patient on the same-day and next-day 64Cu-SAR-bisPSMA PET imaging. Mean SUVmean and SUVmax increased more than 80% (82% and 87% average increase across all readers, respectively) and TBR increased almost 5 times (4.8x average increase across all readers) comparing same-day with next-day imaging (ranges among readers, Day 0 and Day 1, respectively: SUVmean 6.6-9.9 and 14.7-15.8; SUVmax 13.9-14.0 and 22.2-33.4; TBR 23.2-25.4 and 118.1-181.7) (Graphs 1 and 2). Lesions of less than 5 mm in size had SUVmean, SUVmax and TBR value of 16.3, 16.8 and 90.1, respectively (mean values across all readers).
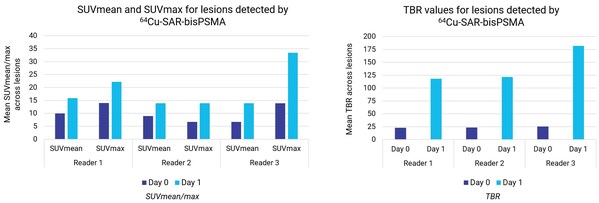
Graph 1, left: SUVmean and SUVmax increased more than 80% from Day 0 to Day 1 (average across all readers). Graph 2, right: TBR increased almost 5 times (4.8x) from Day 0 to Day 1 (average across all readers).
The ability of 64Cu-SAR-bisPSMA to detect lesions less than 5 mm is a result of a few factors unique to the product. 64Cu has a longer half-life (12.7 h) than the isotopes used in currently approved PSMA PET agents, such as gallium-68 (68Ga) and fluorine-18 (18F) (<2 h), enabling next-day imaging, which is impossible with 18F- and 68Ga-based agents. Clarity's SAR Technology holds isotopes of copper securely inside a cage, preventing their leakage when administered to patients. Pre-clinical and clinical evidence has demonstrated that the optimised dual targeting molecule connected to the cage, bisPSMA, ensures increased targeting and retention of the product in PC tumours compared to its single targeting molecule counterpart and approved PSMA agents[9],[10]. Results from Clarity's Phase I PROPELLER trial substantiated this hypothesis as 64Cu-SAR-bisPSMA showed detection of additional lesions and 2-3 times greater uptake within the same PC lesions compared to the generic SOC imaging agent, 68Ga-PSMA-11. Furthermore, the initial positive results from the COBRA study, announced on the 15th of February 2024[2], showed that delayed imaging with 64Cu-SAR-bisPSMA is able to detect PC lesions in up to 80% of patients with BCR of PC, who demonstrated negative or equivocal SOC imaging at screening. It also showed that the number of lesions detected by 64Cu-SAR-bisPSMA almost doubled on delayed imaging compared to same-day imaging.
"The COBRA trial was designed to showcase the true benefits of bisPSMA compared to current SOC imaging, implementing a high standard of study design to validate our findings and utilise an unbiased view of assessment. The results are nothing short of extraordinary, and as we continue to adhere to best practice in clinical development, our science at Clarity is clearly differentiating us from our competitors. Combined with our clinical and pre-clinical evidence to date, this data further validates SAR-bisPSMA as a potential best-in-class PSMA agent for the diagnosis (with 64Cu) and subsequent treatment (with copper-67 [67Cu]) of PC. The benefits of 64Cu-SAR-bisPSMA are now even more evident, given the highest scientific rigour applied to the COBRA study design, and confirmed by clinicians who reported that they would change their treatment plan in response to the 64Cu-SAR-bisPSMA scan results in approximately half of their trial patients. Our COBRA trial was an exploratory first-in-human Phase 1/2 trial in BCR to generate information to allow us to meticulously design a Phase 3 registrational trial. Now that we know what we are looking for, including lesions in the 2 mm range, we can structure a Phase 3 trial in the BCR of PC indication to clearly differentiate ourselves from the first-generation PSMA agents as we head towards our ultimate goal of improving treatment outcomes for people with cancer," Dr Taylor said.
About SAR-bisPSMA
SAR-bisPSMA derives its name from the word "bis", which reflects a novel approach of connecting two PSMA-targeting agents to Clarity's proprietary sarcophagine (SAR) Technology that securely holds copper isotopes inside a cage-like structure, called a chelator. Unlike other commercially available chelators, the SAR Technology prevents copper leakage into the body. SAR-bisPSMA is a Targeted Copper Theranostic that can be used with isotopes of copper-64 (Cu-64 or 64Cu) for imaging and copper-67 (Cu-67 or 67Cu) for therapy.
64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA are unregistered products. The data outlined in this announcement has not been assessed by health authorities such as the US Food and Drug Administration (FDA). A clinical development program is currently underway to assess the efficacy and safety of these products. There is no guarantee that these products will become commercially available.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide[11]. The American Cancer Institute estimates in 2024 there will be 299,310 new cases of prostate cancer in the US and around 35,250 deaths from the disease[12].
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing Targeted Copper Theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com
References
This announcement has been authorised for release by the Executive Chairperson.