Clarity signs copper-67 Supply Agreement with Nusano
SYDNEY, Oct. 16, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce the signing of a Supp...
Co-PSMA trial achieves primary endpoint
SYDNEY, Oct. 14, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce that the Co-PSMA (NCT...
Co-PSMA trial: Recruitment successfully completed
SYDNEY, July 17, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce that the Co-PSMA (NCT...
Clarity enters a Commercial Manufacturing Agreement for Cu-64 SAR-bisPSMA with SpectronRx
Highlights * Clarity has entered into a Commercial Manufacturing Agreement with SpectronRx for64Cu-SAR-bisPSMA. * SpectronRx's facility in Indiana will provide on-demand commercial-scale manufacturing of both copper-64 and64Cu-SAR-bisPSMA under one roof and enables distribution to all 50 sta...
SABRE topline results: Cu-64 SAR-Bombesin is effective in detecting prostate cancer recurrence in patients with negative SOC imaging
Highlights * Topline data from Clarity's diagnostic Phase II trial, SABRE, showed that 64 Cu-SAR-Bombesin was safe, well tolerated and effective at detecting prostate cancer in patients with biochemical recurrence (BCR) who are negative or equivocal on standard-of-care (SOC) scans, including pr...
DISCO topline results: 64Cu-SARTATE is highly effective in detecting tumours in NET patients compared to SOC imaging. Phase III planning underway.
HIGHLIGHTS * Topline data from Clarity's diagnostic Phase II trial, DISCO, confirms that 64Cu-SARTATE is safe and highly effective compared to standard-of-care (SOC) imaging at detecting lesions in patients with neuroendocrine tumours (NETs). * DISCO compared the diagnostic performance of 64C...
First patient imaged in Phase III AMPLIFY trial with 64Cu-SAR-bisPSMA PET/CT
SYDNEY, May 29, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce that it has imaged the...
Registrational Phase III AMPLIFY trial in biochemical recurrence of prostate cancer commences
SYDNEY, May 20, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for patients with cancer, is pleased to announce that it has commenced ...
Clarity signs high-volume commercial-scale copper-64 Supply Agreement with Nusano
Highlights * Clarity has signed a commercial-scale agreement with Nusano, Inc. ("Nusano") for supply of copper-64 (Cu-64 or64Cu) isotope. * The 190,000 square foot Nusano facility in West Valley City, Utah is capable of producing more than 1,000 Ci (37,000 GBq) of copper-64 per day at capaci...
SECuRE trial update: First patient treated in the Phase II Cohort Expansion
HIGHLIGHTS * The first of the planned 24 participants in the Cohort Expansion Phase (Phase II) of the SECuRE trial has been treated with their first dose of 8 GBq of67Cu-SAR-bisPSMA.This follows the recent recommendation by the Safety Review Committee (SRC) after the successful completion of t...
SECuRE trial update: 92% of pre-chemo participants experience greater than 35% drop in PSA levels across all cohorts. Cohort Expansion Phase commences.
HIGHLIGHTS * Safety Review Committee (SRC) meeting confirms end of the Dose Escalation Phase and commencement of the Cohort Expansion Phase (Phase II stage) of the SECuRE study. * Based on the efficacy and safety assessment of all cohorts and the focus on earlier stages of treatment, the SRC...
Clarity receives US FDA Fast Track Designation for the treatment of metastatic castration-resistant prostate cancer patients with Cu-67 SAR-bisPSMA
SYDNEY, Feb. 19, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce thatthe Un...
Clarity receives U.S. FDA Fast Track Designation for Cu-64 SAR-bisPSMA in biochemical recurrence of prostate cancer
SYDNEY, Jan. 24, 2025 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or "Company"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the U...
Clarity expands its pipeline with a novel optimised FAP-targeted radiopharmaceutical
Highlights * Clarity has developed a proprietary fibroblast activation protein (FAP)-targeted radiopharmaceutical product that can be used with the perfect pairing of copper isotopes for the diagnosis and treatment of cancer. * The product, termed SAR-bisFAP, has shown strong tumour targeting...
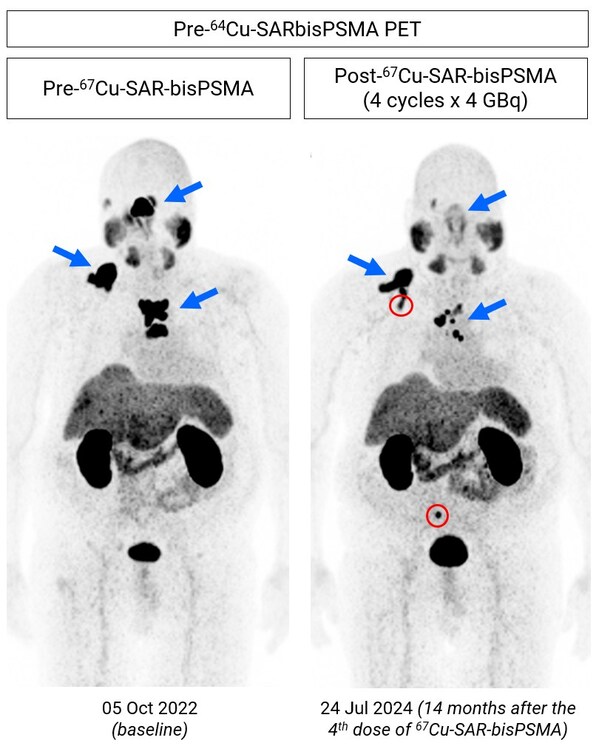
Copper-67 SAR-bisPSMA updates
SYDNEY, Oct. 16, 2024 /PRNewswire/ -- HIGHLIGHTS Cohort 4 - SECuRE Trial * The third participant of cohort 4 (multi-dose) of the SECuRE trial1 has now completed the Dose Limiting Toxicity (DLT) period after a second dose of 12GBq of67Cu-SAR-bisPSMA, following on from the announcement dated 1...
Positive guidance from the U.S. FDA on 64Cu-SAR-bisPSMA Phase III trial in patients with recurrence of prostate cancer
Highlights * United States Food and Drug Administration (U.S. FDA) provided positive feedback on a pivotal Phase III trial for64Cu-SAR-bisPSMA diagnostic in prostate cancer patients with biochemical recurrence (BCR), AMPLIFY. * The positive results of the completed COBRA and PROPELLER trials,...
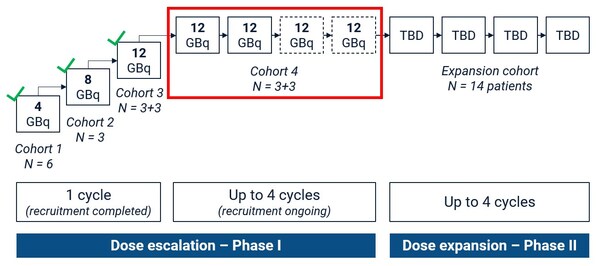
SECuRE trial advances: No dose limiting toxicities and strong preliminary efficacy data in first multi-dose cohort
Highlights * Cohort 4 of the SECuRE trial is the first to assess multiple cycles of 67 Cu-SAR-bisPSMA at the highest dose of 12GBq. * The Safety Review Committee (SRC) assessed early data from the first 3 participants in cohort 4 who received 2 doses of67Cu-SAR-bisPSMA. Two of these participa...
Clarity receives FDA Fast Track Designation for 64Cu-SAR-bisPSMA
SYDNEY, Aug. 22, 2024 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the U.S. Food and ...
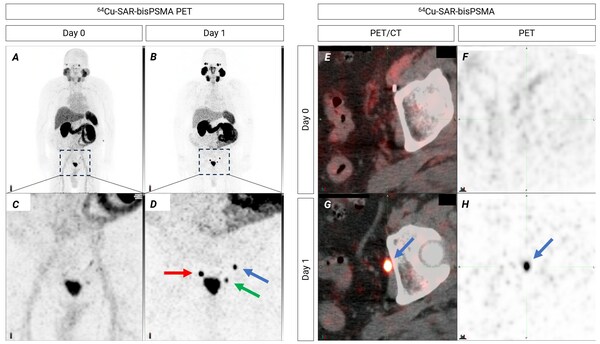
Additional COBRA results: SAR-bisPSMA detects lesions in the 2-millimetre range
Highlights * Clarity recently reported that in its diagnostic Phase 1/2 trial, COBRA, 64 Cu-SAR-bisPSMA was found to be safe and highly effective in detecting prostate cancer (PC) lesions in patients with biochemical recurrence (BCR). * In trial participants where standard of care (SOC) imagin...
Registrational Phase III CLARIFY trial in prostate cancer commences
SYDNEY, Nov. 30, 2023 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce it has commenced its r...