IND approval from the US FDA for theranostic SAR-Bombesin trial in prostate cancer
SYDNEY, Nov. 21, 2022 /PRNewswire/ -- Highlights * Clarity's fifth successful Investigational New Drug (IND) application with theUnited States Food and Drug Administration (US FDA), opening up therapeutic applications for SAR-Bombesin * A total of six products with both the diagnostic and t...
First patient treated in Clarity's therapeutic prostate cancer trial
Highlights * Clarity Pharmaceuticals recruits and treats its first patient in the therapeutic phase of its SAR-bisPSMA theranostic clinical trial SECuRE (NCT04868604)[1] investigating Targeted Copper Theranostics (TCTs) in patients with metastatic castrate-resistant prostate cancer (mCRPC) ...
Recruitment opens for Phase II trial in prostate cancer with Cu-64 SAR-Bombesin in the US
SYDNEY, Sept. 5, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, is pleased to announce that the US-based diagn...
Clarity advances to cohort 3 of the CL04 trial of SARTATE™ in paediatric neuroblastoma
Highlights * Cohort 2 completed in participants with neuroblastoma who received therapy with67Cu SARTATE™ at a dose of 175MBq/kg body weight * No Dose Limiting Toxicities (DLTs) have been reported in cohort 1 and cohort 2 * Safety Review Committee (SRC) has recommended the trial continues w...
Recruitment complete for Clarity's PROPELLER prostate cancer diagnostic trial
SYDNEY, July 20, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce the completion of recruitment for the Phase I PROPELLER diagnosti...
IND approval from the US FDA for Phase II SAR-Bombesin imaging trial in prostate cancer
Highlights * IND approval received for SAR-Bombesin product, enabling a Phase II "SABRE" imaging trial to detect prostate cancer in up to 50 PSMA-negative participants in the US * Approximately 20% of prostate cancer patients with biochemical recurrence (BCR) are PSMA-PET negative and theref...
Dr Neal Shore joins Clarity's Clinical Advisory Board
SYDNEY, May 26, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that DrNeal Shore has joined Clarity's Clinical Advisory Board (C...
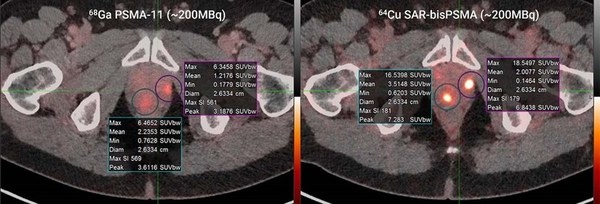
Clarity's US-based Cu-64 SAR-bisPSMA trial in prostate cancer opens for recruitment
SYDNEY, March 28, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that the diagnostic 64Cu SAR-bisPSMA trial (COBRA NCT05249127 <...
New clinical trial collaboration for Cu-64 SAR-bisPSMA in prostate cancer
SYDNEY, March 24, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that an investigator-initiated trial (IIT) will commence shortly...
First patient treated in cohort 2 SARTATE™ neuroblastoma therapy trial
SYDNEY, Feb. 25, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that it has successfully treated its first participant in cohort ...
US FDA Study May Proceed letter for Clarity's Cu-64 SAR-bisPSMA trial in prostate cancer
SYDNEY, Feb. 7, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that it has received a confirmation from the US Food and Drug Adm...
Clarity advances to cohort 2 in the SARTATE™ neuroblastoma trial
SYDNEY, Feb. 1, 2022 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that it has completed cohort 1 and advanced to cohort 2 in the64Cu...
Clarity and Cardinal Health enter into Agreement for Targeted Copper Theranostics
SYDNEY, Dec. 2, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, and Cardinal Health (NYSE: CAH), are pleased to announce that the companies have entered ...
Fifty percent recruitment milestone for PROPELLER prostate cancer trial
SYDNEY, Dec. 1, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, is pleased to announce that 15 of 30 participants have been recruited in the diagnostic64...
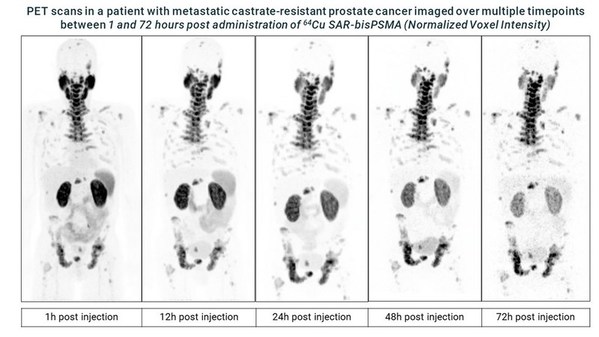
Recruitment for the dosimetry phase of Clarity's Cu-64/Cu-67 SAR-bisPSMA theranostic prostate cancer trial completed
* Clarity Pharmaceuticals completes recruitment for the initial dosimetry phase of its SAR-bisPSMA theranostic clinical trial SECuRE (NCT04868604)[1] investigating Targeted Copper Theranostics (TCT) in patients with metastatic castrate-resistant prostate cancer (mCRPC). * Dosimetry data is be...
Clarity and Evergreen enter Targeted Copper Theranostics manufacturing agreement for US Clinical trials
SYDNEY, Sept. 30, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), an Australian-based clinical stage radiopharmaceutical company developing next-generation products to address the growing need in oncology, and Evergreen Theragnostics, Inc. ("Evergreen"), a radiopharmaceutical...
First patient treated in Clarity's Cu-64/Cu-67 SAR-bisPSMA theranostic prostate cancer trial
SYDNEY, Aug. 25, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or the "Company"), an Australian-based clinical stage radiopharmaceutical company developing next-generation products to address the growing need in oncology, is pleased to announce that the first US patient has ...