Medical/Pharmaceuticals
JW Therapeutics Announces IND Approval for the Pivotal Clinical Trial of Carteyva® in Second-line Large B-Cell Lymphoma
SHANGHAI, March 31, 2022 /PRNewswire/ -- JW Therapeutics (HKEx: 2126), an independent, innovative biotechnology company focused on developing, manufacturing and commercializing cell immunotherapy products, announced that it has received the Investigational New Drug (IND) clearance from the Nation...
CStone announces first patient enrollment in the U.S. in the Phase 1 clinical trial of CS5001, a potential global best-in-class ROR1-targeting ADC
* Commencement of the first-in-human clinical trial of CS5001 marks another key milestone for CStone's Pipeline 2.0 * Global development of CS5001 is conducted in the form of a multi-regional clinical trial, with sites initiated in the US andAustralia, and an IND application accepted by the ...
Everest Medicines Submits New Drug Application in Hong Kong for Sacituzumab Govitecan in Second-Line Metastatic Triple-Negative Breast Cancer
SHANGHAI, March 31, 2022 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK), a biopharmaceutical company focused on developing and commercializing transformative pharmaceutical products to address critical unmet needs inAsia Pacific markets, announced today that it has submitted a New Drug Applicat...
Change of Composition of the Telix Board of Directors
* Non-Executive Director Oliver Buck to retire at the Annual General Meeting * Tiffany Olson, experienced U.S.-based pharma executive appointed as independent Non-Executive Director MELBOURNE, Australia and INDIANAPOLIS, March 31, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, T...
Simulare Medical, a Division of Smile Train, Announces Patent-Pending Bilateral Cleft Lip and Palate Simulator
New Simulator to Accelerate the Learning Curve in Cleft Reconstructive Surgical Training & Ultimately Further Care for Cleft-Affected Children FORT WORTH, Texas, March 31, 2022 /PRNewswire/ -- Simulare Medical, a Division of Smile Train, Inc., has launched the newest innovation in its growing li...
LifeTech Scientific Corporation(1302.HK) Announces 2021 Annual Results
Excluding certain non-recurring items, the net profit attributable to owners of the Company increased by 76.8% to RMB 324.0million SHENZHEN, China, March 30, 2022 /PRNewswire/ -- LifeTech Scientific Corporation (LifeTech, 1302.HK), a leading company specialized in minimally invasive interventio...
Keymed Biosciences Releases 2021 Annual Results
CHENGDU, China, March 30, 2022 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX Code: 02162) released 2021 annual results. BUSINESS HIGHLIGHTS On July 8, 2021, Keymed was listed on the HKEX. During the Reporting Period, the Company continued proceeding the R&D of products and made the following p...
Broncus Medical (02216.HK) Announces Annual Results for 2021: Operating revenue for 2021 increased 234.2% year-on-year, while product development and commercialization are steadily advancing.
HANGZHOU, China, March 30, 2022 /PRNewswire/ -- On March 29, 2022, Broncus Medical (02216.HK), the leader in precision interventional treatment for lung diseases inChina, announced its annual results announcement for the year ended December 31, 2021. During the period, the Group earned revenue of...
Symbiance announces the launch of SimpleStats, an application for statistical analysis and reporting of clinical data
PRINCTON, N.J., March 30, 2022 /PRNewswire/ -- SimpleStats (www.SimpleStats.ai) is designed to help Pharmaceutical/Biotech industries to automate statistical analysis and reporting of clinical data! Symbiance announced the launch of its Artificial Intelligence (AI) powered product SimpleStats. T...
Transcenta Announces Presentation of Preclinical Data of TST005 at the 2022 American Association for Cancer Research (AACR) Annual Meeting
SUZHOU, China, March 30, 2022 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, announces the preclinical data...
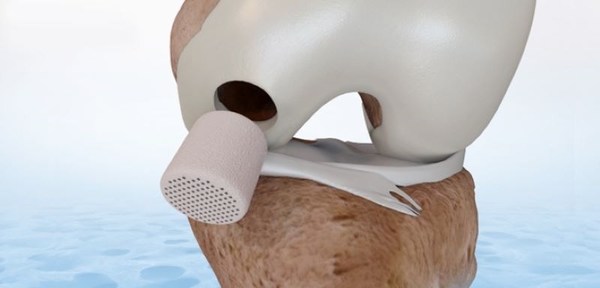
FDA approves CartiHeal's Implant for the Treatment of Cartilage and Osteochondral Defects
Approval is based on results of a multicenter, randomized, open-labeled and controlled IDE clinical study that demonstrated the superiority of Agili-C™ implant to the current surgical standard of care, debridement and microfracture in the knee joint KFAR SABA, Israel, March 30, 2022 /PRNewswire/...
Ascletis Announces Completion of Patient Enrollment in Phase II Clinical Trial of ASC42, an In-House Developed, Best-in-Class FXR Agonist for Chronic Hepatitis B Indication
--Compared to the placebo cohort, both 10 mg and 15 mg ASC42 cohorts are safe and well tolerated to date. Most of adverse events are grade 1 or 2. --ASC42 is a novel anti-viral candidate for HBV functional cure through inhibiting the transcription of HBV cccDNA into HBV RNA and reducing the HBV ...
BioVaxys Expands Cancer Vaccine Platform
BVX-0922 to Target Colorectal Cancer Under Investigator-Sponsored IND VANCOUVER, BC, March 30, 2022 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV), (FRA: 5LB), (OTCQB: BVAXF) ("BioVaxys" or "Company"), announced today the expansion of its cancer vaccine platform with BVX-0922, its autolog...
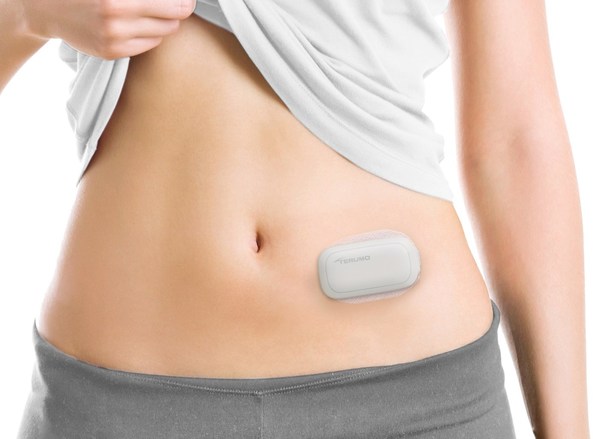
Terumo and Glooko Announce Collaboration on New Solution for Diabetes Data Sharing
A Technology Integration for MEDISAFE WITHTM Insulin Patch Pump Patients to
Upload Data Anytime Anywhere
TOKYO, March 30, 2022 /PRNewswire/ -- Terumo Corporation (TSE: 4543), a
global leader in medical technology, andGlooko
GILEAD ANNOUNCES NEW DATA REINFORCING CLINICAL BENEFITS OF ITS ANTIVIRAL TREATMENTS AMONG PEOPLE LIVING WITH HEPATITIS B AND C IN ASIA (APASL 2022)
* Studies show potential improvement in risk of liver cancer in chronic hepatitis B patients after long-term antiviral treatment * Improved renal and bone parameters with tenofovir alafenamide (TAF) compared to tenofovir disoproxil fumarate (TDF) across multiple chronic hepatitis B patient t...
First Year of Commercialization, Alphamab Oncology Reports Full Year 2021 Financial Results and Business Highlights
SUZHOU, China, March 30, 2022 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) reported financial results for the full year endedDecember 31, 2021 and highlighted recent progress and upcoming milestones. Highlights * First product launch: In November 2021, KN035 (Envafolimab) has receiv...
First Year of Commercialization, Alphamab Oncology Reports Full Year 2021 Financial Results and Business Highlights
SUZHOU, China, March 29, 2022 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) reported financial results for the full year endedDecember 31, 2021 and highlighted recent progress and upcoming milestones. Highlights * First product launch: In November 2021, KN035 (Envafolimab) has receiv...
Antengene Announces IND Approval in China for a Phase II Study of Eltanexor (ATG-016) in Patients with High-Risk Myelodysplastic Syndromes
* This study is the third ATG-016 study to be conducted in China. * Study highlights Antengene's robust SINE programs. SHANGHAI and HONG KONG, March 30, 2022 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), a leading innovative, commercial-stage global biopharmaceutic...
RNAimmune Secures $27 Million Series A Round of Financing for Development of mRNA Therapeutics and Vaccines
GAITHERSBURG, Md., March 30, 2022 /PRNewswire/ -- RNAimmune, Inc. (the "Company " or "RNAimmune"), a biopharmaceutical company specializing in discovery and development of mRNA-based therapeutics and vaccines, announced today that is has secured an approximatelyUS$27 million Series A round of fina...
Brii Bio Announces Strategic Partnership with Sinopharm to Advance Commercialization of Long-Acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, in China
DURHAM, N.C. and BEIJING, March 30, 2022 /PRNewswire/ -- Brii Biosciences
Limited
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 272 media titles]
2024-04-24 17:09