Medical/Pharmaceuticals
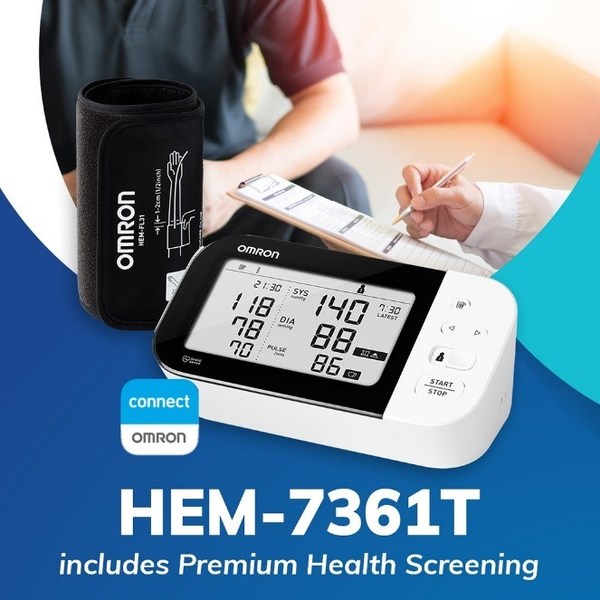
OMRON and Doctor Anywhere announce collaboration for digitally enhanced remote hypertension and health management program
* Initiative to strengthen OMRON's vision of 'Going For Zero' (heart attacks and strokes). SINGAPORE, Dec. 6, 2021 /PRNewswire/ -- OMRON Healthcare, the global leader in the field of clinically proven and innovative medical equipment for health monitoring and therapy has announced a strategic ...
Jacobio Receives IND Approval for Combination Therapy of KRAS G12C and Cetuximab Injection in China
BEIJING and SHANGHAI and BOSTON, Dec. 5, 2021 /PRNewswire/ -- Jacobio Pharma ( 1167.HK) has received the Investigational New Drug (the "IND") approval of the combination therapy of KRAS G12C inhibitor JAB-21822 and Cetuximab injection from the Center for Drug Evaluation ofChina (the "CDE") on Dec...
Kazia Announces Positive Final Data From Phase II Clinical Study Of Paxalisib In Newly Diagnosed Glioblastoma
SYDNEY, Dec. 4, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce positive final data from a phase II clinical study of paxalisib as first line therapy in patients with glioblastoma (NCT03522298). The res...
Henlius' 4th Biologics: Bevacizumab Biosimilar Hanbeitai Approved by National Medical Products Administration
SHANGHAI, Dec. 4, 2021 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that bevacizumab biosimilar Hanbeitai, developed and manufactured by Henlius independently, has been approved by the National Medical Products Administration (NMPA). It is indicated for the treatment of meta...
I-Mab Announces First Two Patients Dosed in U.S. Phase 2 Combination Trial of Uliledlimab with Atezolizumab in Patients with Selected Advanced Solid Tumors
SHANGHAI and GAITHERSBURG, Md., Dec. 3, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that the first two patients have been dosed in the U.S. p...
OrigiMed announced its strategic partnership with Janssen to develop clinical innovative solutions driven by data science
SHANGHAI, Dec. 3, 2021 /PRNewswire/ -- OrigiMed Co., Ltd. ("OrigiMed") announced that the company has signed a memorandum of understanding (MoU) on strategic collaboration with Janssen (China) Research and Development Center of Johnson & Johnson (China) Investment Co., Ltd. ("Janssen").Zili Li, M...
Biotheus' Bifunctional Therapeutic PM8001 has been Approved by the USFDA to Enter Phase II Studies
ZHUHAI, China, Dec. 3, 2021 /PRNewswire/ -- Biotheus Inc., has announced that its proprietary anti-PD-L1/TGF-β bifunctional therapeutic, named PM8001, has been approved by the USFDA (United States Food and Drug Administration) for conducting clinical phase II studies as part of an international m...
Mongolian Government To Build On COVID-19 Vaccine And Booster Success
* Mongolia secures nearly 70% vaccination rates and an overall casualty rate of only 0.3% * Successes against COVID-19 position country well for ambitious and progressive reforms as part of government's Vision 2050 agenda ULAANBAATAR, Mongolia, Dec. 3, 2021 /PRNewswire/ -- Mongolia is prepari...
Elekta's new sustainability-linked bond oversubscribed
STOCKHOLM, Dec. 3, 2021 /PRNewswire/ -- Elekta (EKTA-B.ST) announced today that it has issuedSEK 1.5 billion senior unsecured sustainability-linked bonds under its Medium Term Note Program (MTN-Program). One of the bonds, in the amount of SEK 1,150 million, has a maturity of 5 years with a coupon ...
RemeGen's Telitacicept and Disitamab Vedotin Enter China's National Reimbursement Drug List for SLE and Gastric Cancer Treatment
YANTAI, China, Dec. 3, 2021 /PRNewswire/ -- RemeGen Co., Ltd. (9995.HK), a commercial-ready biotechnology company, today announced that two of its innovative drugs, a dual-targeted TACI-Fc fusion protein, Telitacicept (RC18) for treating systemic lupus erythematosus (SLE) and a novel antibody-dru...
Innovent and Lilly Announce Successful Expansion of Sintilimab in China National Reimbursement Drug List to Include Three Additional First-Line Indications
SAN FRANCISCO, INDIANAPOLIS, and SUZHOU, China, Dec. 3, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune and ...
Ascletis Announces Inclusion in New Catalogue of China National Reimbursement Drug List (NRDL) of ASCLEVIR®/ GANOVO® Regimen, an All-oral Direct Anti-HCV Therapy
HANGZHOU, China and SHAOXING, China, Dec. 3, 2021 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672) today announces that its all-oral direct anti-hepatitis C virus (HCV) ASCLEVIR® (Ravidasvir)/ GANOVO® (Danoprevir) regimen has been included in the Medicine Catalog for National Basic Medical Insu...
InnoCare Announces Inclusion of Orelabrutinib in China National Reimbursement Drug List
BEIJING, Dec. 2, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a commercial-stage biotech company, announced today that its BTK inhibitor orelabrutinib has been included in the updated National Reimbursement Drug List (NRDL) by the China National Healthcare Security Administration (NHSA). ...
Decode Genetics Publishes the Largest Ever Study of the Plasma Proteome
REYKJAVIK, Iceland, Dec. 3, 2021 /PRNewswire/ -- In a study published today in Nature genetics, scientists at deCODE genetics , a subsidiary of the pharmaceutical company Amgen, demonstrate how measuring the levels of a large number of proteins in plasma at population scale when combined with dat...
Hepagene Therapeutics Initiates the RISE Study, a Phase IIa Clinical Trial of HPG1860 in Patients with NASH
SHANGHAI, Dec. 2, 2021 /PRNewswire/ -- Hepagene Therapeutics, Inc, a clinical stage biopharmaceutical company focusing on innovative therapies for patients with liver diseases, today announced that it has screened the first patient in theUSA for the RISE study, a Phase IIa clinical trial of HPG18...
MingMed Biotechnology Announces U.S. FDA Approval for IND Application of HPK1 Small Molecule Inhibitor PRJ1-3024, and Completion of Phase I Clinical Trials for Dry AMD Treatment Drug QA102
GUANGZHOU, China, Dec. 2, 2021 /PRNewswire/ -- MingMed Biotechnology, a clinical stage company dedicated to developing first-in-class pharmaceutical products, announced recently the US Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for PRJ1-3024, a ...
Clarity and Cardinal Health enter into Agreement for Targeted Copper Theranostics
SYDNEY, Dec. 2, 2021 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical-stage radiopharmaceutical company developing next-generation products to address the growing needs in oncology, and Cardinal Health (NYSE: CAH), are pleased to announce that the companies have entered ...
Rayence's Low-Dose Detector "GreenON" Receives Critical Acclaim at RSNA 2021
SEOUL, South Korea, Dec. 2, 2021 /PRNewswire/ -- Rayence(www.rayence.com
Joint CUHK-HKU study discovers efficacy of COVID-19 vaccines correlates with a probiotic bacterium, Bifidobacterium adolescentis
HONG KONG, Dec. 2, 2021 /PRNewswire/ -- The Centre for Gut Microbiota Research of the Faculty of Medicine at TheChinese University of Hong Kong (CU Medicine) is among the first in the world to discover that germs in our gut (gut microbiota) modulate our immunity and disease severity in COVID-19. ...
DiNovA Medtech Announces Mr. Yaniv Kirma to Join as DiNovA Israel Incubator's Chief Operating Officer
CAESAREA, Israel, Dec. 2, 2021 /PRNewswire/ -- DiNovA Medtech, a leading medical devices incubator inChina ("DiNovA"), is pleased to announce Mr. Yaniv Kirma, to come on board as Chief Operating Officer of DiNovA Israel Incubator ("DiNovA Israel"). Mr. Kirma will join force with Chief Medical Off...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 273 media titles]
2024-04-24 17:09Introducing Wacom Movink: The first OLED pen display, and the thinnest and lightest Wacom pen display ever
[Picked up by 268 media titles]
2024-04-24 13:00