Medical/Pharmaceuticals
Lynk Pharmaceuticals Announced First Patient Dosed in Phase II Clinical Study of LNK01001
HANGZHOU, China, Dec. 1, 2021 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, announced the company has dosed the first patient with LNK01001 in its Phase II clinical trial in subjects with rheumatoid arthri...
Everest Medicines and Providence Therapeutics Jointly Announce Vaccine Development Strategy to Address Omicron (B.1.1.529) SARS-CoV-2 Variant
SHANGHAI and CALGARY, Canada, Dec. 1, 2021 /PRNewswire/ -- Everest Medicines and Providence Therapeutics ("Providence") jointly announced today that the companies have started working on a new version of COVID-19 vaccine specifically targeting the new Omicron variant. Scientists from the two com...
Innovent Biologics and Ascentage Pharma Announce the China NMPA Approval for China's First Third-Generation BCR-ABL Inhibitor Olverembatinib for the Treatment of Chronic Myeloid Leukemia
SUZHOU, China, SAN FRANCISCO and ROCKVILLE, Md., Dec. 1, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune and...
EpiVax Progresses on a Vaccine to Address SARS-CoV-2 Variants
PROVIDENCE, R.I., Dec. 1, 2021 /PRNewswire/ -- Today EpiVax, Inc. ("EpiVax") confirmed that the company'sEPV-CoV-19 vaccine epitopes are 98.2% conserved in the new Omicron SARS-CoV-2 variant. EpiVax and EpiVax Therapeutics, Inc. ("EVT") are also affirming commitment to move the novel T cell epito...
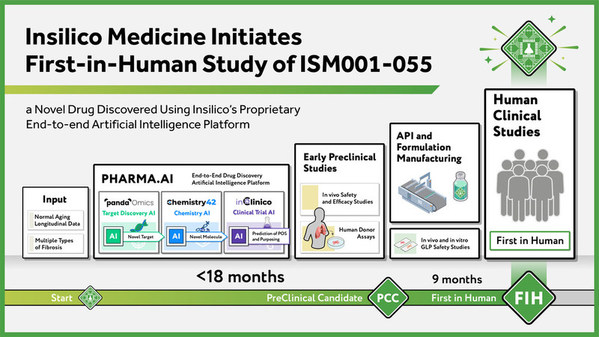
Insilico Medicine Initiates First-in-Human Study of ISM001-055, a Novel Drug Discovered Using Insilico's Proprietary End-to-end Artificial Intelligence Platform
NEW YORK and HONG KONG, Dec. 1, 2021 /PRNewswire/ -- Insilico Medicine, an
end-to-end artificial intelligence (AI)-driven drug discovery company,today
announced that the first healthy volunteer has been dosed in a first-in-human
microdose trial of ISM001-055.
RedHill Biopharma Reports Operational Highlights and Third Quarter 2021 Financial Results
RedHill Accelerates its Two Advanced COVID-19 Pill Clinical Programs in Light of their Potential Against Omicron Acting independently of spike protein mutation, opaganib and RHB-107's unique host-targeted mechanisms of action hold potential versus Omicron and other variants -- Phase 2/3 study s...
New CELLSEARCH® Circulating Multiple Myeloma Test now available to help community physicians optimize patient care
As the market leader in cell-based liquid biopsies, Menarini Silicon Biosystems is offering community hematology-oncologists an opportunity to have patient blood samples sent to a US centralized laboratory where itsCELLSEARCH® technology can enumerateCirculating Multiple Myeloma Cells (CMMCs) f...
Standigm signs MOU with Institut Pasteur Korea for AI-based drug discovery research on infectious disease
SEOUL, South Korea, Nov. 30, 2021 /PRNewswire/ -- Standigm Inc. ("Standigm"), the leading workflow artificial intelligence (AI) drug discovery company, today announced the signing of a Memorandum of Understanding ("MOU") with Institut Pasteur Korea ("IPK"), the infectious disease-focused research...
NeoLight Acquires Occular Screening Leader Phoenix Technology Group, Strengthening the Company's Newborn Care Global Strategy
SCOTTSDALE, Ariz., Nov. 30, 2021 /PRNewswire/ -- NeoLight LLC (NeoLight), an ASU spinout and anArizona based newborn medical device company, today announced it has acquired Phoenix Technology Group LLC (PTG), a leading provider of advanced ophthalmic imaging devices, including a telemedicine plat...
Jemincare Announces the Exclusive License of the Kras Inhibitor to HUYABIO
SHANGHAI, Nov. 30, 2021 /PRNewswire/ -- Jemincare, a leading pharmaceutical company fromChina, announced today its wholly owned subsidiary company, Shanghai Jemincare Pharmaceutical Co., Ltd., had licensed exclusive worldwide ex-China rights to the Kras inhibitor, JMKX1899, to HUYABIO Internation...
George Clinical Applauded by Frost & Sullivan for Improving Clinical Trial Efficiency and Patient-centricity through Impactful, Strategic Partnerships
Innovation through impactful, strategic partnerships improves efficiency and delivers scientific leadership and patient-centricity in clinical trials execution. SANTA CLARA, Calif., Nov. 30, 2021 /PRNewswire/ -- Based on its recent analysis of theAsia-Pacific clinical contract research organizat...
Treadwell Therapeutics Announces US FDA Clearance of IND Application for Phase 2 Study of TTK inhibitor, CFI-402257
NEW YORK and HONG KONG, Nov. 30, 2021 /PRNewswire/ -- Treadwell Therapeutics, a clinical stage biotechnology company developing novel therapeutics for highly aggressive cancers, today announced that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) applic...
Seegene's High Multiplex PCR Assay Capable of Detecting New Omicron Variant
SEOUL, South Korea, Nov. 30, 2021 /PRNewswire/ -- Seegene Inc. (KQ 096530), South Korea's leading molecular diagnostics company, today confirmed that its Allplex™ SARS-CoV-2 Master Assay is capable of detecting the Omicron's unique pattern of mutations. The well-established PCR-based test recogni...
JOYSBIO's SARS-COV-2 Antigen Rapid test detects OMICRON variant
TIANJIN, China, Nov. 30, 2021 /PRNewswire/ -- JOYSBIO, one of the world's leading manufacturers of lateral flow rapid tests, is proud to announce that their tests are available around the world. Furthermore, they've been able to show that their test can detect the new Omicron (B.1.1.529). This va...
ABM Therapeutics Receives IND Approval in China for BRAF Inhibitor ABM-1310
SAN DIEGO and SHANGHAI, Nov. 30, 2021 /PRNewswire/ -- ABM Therapeutics (ABM), a clinical-stage biopharmaceutical company with a focus on treating brain cancers and cancer metastases, today announced that its IND application for ABM-1310, a new-generation BRAF inhibitor, has been approved by the N...
Mayne Pharma and Mithra Announce TGA Approval of NEXTSTELLIS® Oral Contraceptive
ADELAIDE, Australia, Nov. 30, 2021 /PRNewswire/ -- Mayne Pharma Group Limited (ASX: MYX) and Mithra Pharmaceuticals, SA (Euronext Brussels: MITRA) are very pleased to announce the Therapeutic Goods Administration (TGA) has approved the novel combined oral contraceptive NEXTSTELLIS® (14.2 mg of es...
Sciwind Biosciences Acquires Global, Exclusive Rights to Develop and Commercialize Sanofi's GIP Receptor Agonists for the Treatment of Metabolic Disease
HANGZHOU, China and SAN FRANCISCO, Nov. 30, 2021 /PRNewswire/ -- Sciwind Biosciences Co., Ltd., a clinical-stage biopharmaceutical company focused on discovering and developing innovative therapies to treat metabolic disease, announced today that it has signed a global, exclusive license agreemen...
SOPHiA GENETICS Hosts Symposium with GE Healthcare at RSNA 2021
Symposium to address how new AI-powered analytic and workflow solutions for clinical and biopharma markets are advancing data-driven medicine Symposium encapsulates SOPHiA GENETICS and GE Healthcare alliance to provide joint solutions and multimodal analytics to healthcare providers and biopharma...
Q-Sera enters exclusive agreement with Terumo Corporation for RAPClot™ rapid serum blood collection tube technology in Japan
MELBOURNE, Australia, Nov. 29, 2021 /PRNewswire/ -- Q-Sera Pty Ltd, an Australian venture-backed company, today announced a partnership with Terumo Corporation, the leading Japanese medical device company, which will see its patent protected RAPClot™ rapid serum tube technology manufactured and u...
GBM AGILE Opens to Paxalisib in Canada
SYDNEY, Nov. 29, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an oncology-focused drug development company, is pleased to inform stakeholders that the GBM AGILE study in glioblastoma (NCT03970447) has opened at Sunnybrook Health Sciences Centre inToronto, Ontario. Thi...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 273 media titles]
2024-04-24 17:09Introducing Wacom Movink: The first OLED pen display, and the thinnest and lightest Wacom pen display ever
[Picked up by 268 media titles]
2024-04-24 13:00