Pharmaceuticals
Akeso Announced 2023 Interim Results: First Profit, Growing Sales of PD-1/CTLA-4 Bispecific Antibody and Priority Review of PD-1/VEGF
—During the reporting period, Akeso's revenue was RMB3,676.9 million, an increase of 2,154.4% from 2022H1; Akeso recorded a profit ofRMB2,489.5 million. —RMB2,915.2 million was recognized by Akeso as license fee income during the reporting period due to the receipt of an upfront payment equivalen...
Cerecin to Present New Infantile Spasms Data at the 35th International Epilepsy Congress, Dublin 2023
* Data from the pilot study in infantile spasms showed positive outcomes. * The results will be presented by Cerecin's Chief Medical Officer, Dr. Marc Cantillon on Sunday 2nd September at 2pm (GMT+1). * The Cerecin team will be accompanied by PharmaVentures, who will be supporting Cerecin's ...
Cerecin to Present New Infantile Spasms Data at the 35th International Epilepsy Congress, Dublin 2023
* Data from the pilot study in infantile spasms showed positive outcomes. * The results will be presented by Cerecin's Chief Medical Officer, Dr. Marc Cantillon on Sunday 2nd September at 2pm (GMT+1). * The Cerecin team will be accompanied by PharmaVentures, who will be supporting Cerecin's ...
Viva Biotech (1873.HK) Announces 2023 Interim Results:Solid Growth in Main Business, Significant Rebound in Profitability
Highlights of the Interim Results as of June 30, 2023: * Revenue reached RMB1,142.2 million, representing a year-on-year increase of approximately 3.0% * Gross profit amounted to RMB406.0 million, representing a year-on-year increase of approximately 17.7% * Adjusted Non-IFRS net profit amo...
Akeso Announced Completion of Patient Enrollment in Phase 3 Trial of Ivonescimab (PD-1/VEGF) versus Pembrolizumab in First-line PD-L1 Positive Advanced NSCLC
HONG KONG, Aug. 29, 2023 /PRNewswire/ -- Akeso Inc. ("Akeso", 9926. HK) announced completion of patient enrollment in a head-to-head study of ivonescimab (AK112, PD-1/VEGF bispecific antibody) compared with pembrolizumab as first-line treatment for patients with PD-L1 positive (PD-L1 TPS≥1%) loca...
Bayer Launches Stunting Prevention Program "CETING" for Community of Cisalak, Depok
DEPOK, Indonesia, Aug. 29, 2023 /PRNewswire/ -- Bayer, a global Life Science company in health and nutrition, is taking an active role in addressing the stunting problem inIndonesia. Coinciding with Bayer's 66th year in Indonesia, Bayer launched the CETING (Prevent Stunting) program for the commu...
ProfoundBio to Participate in the 21st Annual Morgan Stanley Conference
SEATTLE, Aug. 28, 2023 /PRNewswire/ -- ProfoundBio, a clinical-stage biotechnology company focused on the development of novel antibody-drug conjugate therapeutics for cancer, announced that management will be participating in one-on-one meetings at the Morgan Stanley 21st Annual Global Healthca...
Ferinject® granted upgraded recommendations in 2023 ESC heart failure guidelines
The 2023 European Society of Cardiology (ESC) guidelines for acute and chronic heart failure (HF) include upgraded recommendations for intravenous (IV) iron supplementation, including Ferinject® (ferric carboxymaltose), for the management of iron deficiency in patients with HF. Phase IV HEART-FI...
Harbour BioMed Announces 2023 Interim Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 28, 2023 /PRNewswire/ -- Harbour BioMed ("HBM", or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology an...
Harbour BioMed Announces US IND Clearance of Its First ADC Program HBM9033 in Solid Tumors
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 28, 2023 /PRNewswire/ -- Harbour BioMed (the"Company", HKEX: 02142) announced that the U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application to commence clinical trials of its first antib...
Jiangsu Recbio Technology Co., Ltd. announced 2023 interim results report and latest progress
TAIZHOU, China, Aug. 28, 2023 /PRNewswire/ -- Innovative vaccine company Recbio (02179.HK) announced its latest progress and interim results for 2023. During the reporting period, the company always adhered to the mission of " Protect Human Health With Best-In-Class Vaccines", rapidly promoted pr...
Specialised Therapeutics acquires commercialisation rights to new oral MND therapy
.. ST to partner with Dutch biotech company Treeway BV .. First CNS therapy in ST therapeutic portfolio SINGAPORE, Aug. 27, 2023 /PRNewswire/ -- Independent biopharmaceutical company Specialised Therapeutics Asia Pte Ltd (ST) will partner withNetherlands based biotechnology company Treeway BV to...
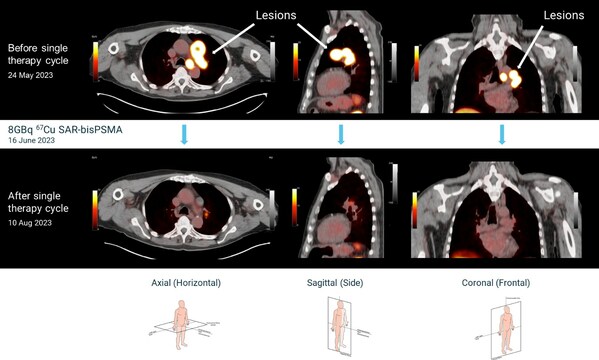
First participant treated at the highest dose level in Clarity's theranostic prostate cancer trial
Highlights * First participant of cohort 3 in the theranostic SECuRE trial investigating 64Cu/67Cu SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has been treated at the highest dose level of 12GBq. * Cohort 2 was recently completed in 3 participants who received therapy...
Akeso Announced that Ivonescimab Granted Priority Review of NDA by China NMPA
HONG KONG, Aug. 25, 2023 /PRNewswire/ -- Akeso Inc. ("Akeso", the "Company"; 9926.HK), a commercial-stage biopharmaceutical company focused on developing and commercializing first-in-class and best-in-class innovative medicines globally, announced today that the National Center for Drug Evaluatio...
Antengene Announces Interim Financial Results for 2023 with New Clinical Data Highlighting the Growing Value of Its Pipeline
SHANGHAI and HONG KONG, Aug. 25, 2023 /PRNewswire/ -- Antengene Corporation Limited ("Antengene" SEHK: 6996.HK), today announced its interim results for the six-months endedJune 30, 2023, and provided updates on multiple milestones achieved since the beginning of 2023. Dr. Jay Mei, Antengene's F...
Henlius 2023 H1 Results: Achieved record profits and RMB2.5 billion revenues, opening a new chapter of high-quality growth
SHANGHAI, Aug. 25, 2023 /PRNewswire/ -- Henlius (2696.HK) announced its 2023 interim results. In the first half of 2023, Henlius reported a boost in operating profits ofRMB240.0 million and revenues of RMB2.5005 billion, up by 93.9% YoY, driven by growth in core oncology products. HANQUYOU (trast...
Tigermed Reports 2023 Interim Results
HANGZHOU, China, Aug. 25, 2023 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. ("Tigermed" or the "company") (Stock code: 300347.SZ / 3347.HK), a leading provider of clinical research solutions across full lifecycle of global biopharmaceutical and medical device products, announced its int...
GC Biopharma to Produce Cholera Vaccines Jointly with Eubiologics
YONGIN, South Korea, Aug. 25, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a South Korean biopharmaceutical company, announced today that it has signed a MOU at its headquarters in Yongin,South Korea with Eubiologics for a co-production of Euvichol, an oral cholera vaccine. Under the MOU, bo...
3SBio announces 2023 interim results, with revenue growing over 20% year on year and pipeline value constantly enhanced
HONG KONG, Aug. 25, 2023 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today released its 2023 interim results. Revenue in the first half of 2023 reached approximatelyRMB3,783.8 million, up 22.3% on a yearly basis. Gross profit was approximatelyRMB3,201.6 million, up ...
Everest Medicines Announces New Drug Application Acceptance by the Pharmaceutical Administration Bureau of Macao for Nefecon® for the Treatment of Primary IgA Nephropathy
SHANGHAI, Aug. 25, 2023 /PRNewswire/ -- Everest Medicines
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 273 media titles]
2024-04-24 17:09Introducing Wacom Movink: The first OLED pen display, and the thinnest and lightest Wacom pen display ever
[Picked up by 268 media titles]
2024-04-24 13:00