Pharmaceuticals
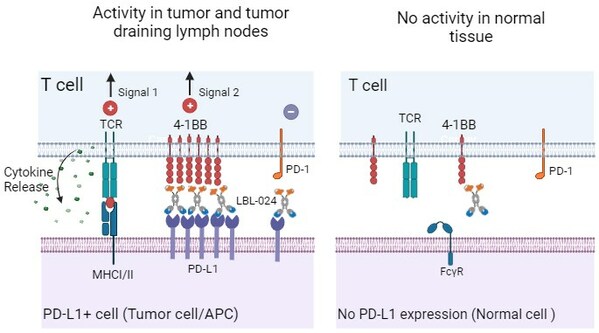
A Potential First-In-Class Drug: CDE Approved Single-Arm Pivotal Clinical Study of LBL-024, An Anti-PD-L1/4-1BB Bispecific Antibody Developed by Leads Biolabs
NANJING, China, April 30, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to co...
Medison Pharma and Alnylam Pharmaceuticals Announce Expansion of their Multi-Regional Partnership in Europe and Israel to Commercialize RNAi Therapeutics in additional LATAM and APAC markets including Australia
* The expanded partnership will allow Alnylam and Medison to help accelerate access for patients in multiple regions under one global alliance * Medison, the creator and leader of the multi-regional partnership category, will utilize its unique, unifiedplatform for efficient global commerciali...
HanAll Biopharma Reports Q1 2024 Financial Results and Provides Business Update
* Delivered solid performance to start 2024, with record-breaking first quarter revenue of34.1 billion KRW. Strong sales momentum continued from key products, funding investments in ongoing R&D programs. * Phase 3 VELOS-4 study of tanfanercept in dry eye disease expected to be initiated in th...
I-MAB Filed 2023 Annual Report on Form 20-F
ROCKVILLE, Md., April 30, 2024 /PRNewswire/ -- I-Mab (the "Company") (NASDAQ: IMAB), a U.S.-based, global biotech company, exclusively focused on the development and potential commercialization of highly differentiated immunotherapies for the treatment of cancer, today announced that it has filed...
111 to Announce First Quarter 2024 Unaudited Financial Results on May 23, 2024 - Conference Call to Follow
SHANGHAI, April 30, 2024 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced that it will report its unaudited financial result...
Innovent Announces the Appointment of Dr. Samuel Zhang as Global Chief Business Officer (CBO)
SAN FRANCISCO and SUZHOU, China, April 30, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, cardiovascular and metabolic, autoimmune,...
WuXi Biologics Releases 2023 ESG Report Demonstrating Strong Sustainability Commitment
* The Company demonstrated a deep commitment to achieving ESG success in partnership with global clients, creating long-term value for all stakeholders. * The Company made remarkable progress in tackling climate change, achieving a 29% intensity reduction of Scope 1 and Scope 2 greenhouse gas ...
Chang Gung Memorial Hospital, Linkou, Garners Two Prestigious International Awards for Its Outstanding Achievements
The hospital honored with Hospital of the Year - Taiwan and Smart Hospital Initiative of the Year -Taiwan at the Healthcare Asia Awards TAOYUAN, April 30, 2024 /PRNewswire/ -- The Healthcare Asia Awards, a prestigious international recognition program, honors exceptional healthcare institutions ...
Henlius Trastuzumab Receives FDA Approval in the United States
SHANGHAI, April 29, 2024 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the company's business partner, Accord BioPharma Inc. (the U.S. specialty division of Intas Pharmaceuticals, Ltd.), has received approval from the United States Food and Drug Administration (FDA) for ...
YS Biopharma Receives Additional 180 Day Extension by Nasdaq to Regain Compliance with Minimum Bid Price Rule
GAITHERSBURG, Md., April 29, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious dise...
Technoderma Medicines Advances TDM-180935 Atopic Dermatitis Clinical Program with Phase 2 Trial
CHENGDU, China, April 29, 2024 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report the Company has begun dosing patients in its Phase 2a clinical trial (NCT06363461) of topical TDM-180935 ointment. This clinical trial in t...
YS Biopharma Receives Additional 180 Day Extension by Nasdaq to Regain Compliance with Minimum Bid Price Rule
GAITHERSBURG, Md., April 29, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious dise...
Prota Therapeutics' CEO Mimi Tang honoured at BioMelbourne Network's Women in Leadership Awards 2024
MELBOURNE, Australia, April 29, 2024 /PRNewswire/ -- Prota Therapeutics' CEO ProfessorMimi Tang has been honoured in the prestigious 2024 BioMelbourne Network Women in Leadership Awards. Professor Tang's exceptional work as a lead researcher for the Murdoch Children's Research Institute (MCRI) an...
DP Technology DevDay 2024 Showcases Large Science Models and Announces Open Science Initiative
BEIJING, April 29, 2024 /PRNewswire/ -- In recent years, the rapid development of artificial intelligence has introduced new possibilities across numerous scientific disciplines. As an AI for Science pioneer, DP Technology is continually collaborating with partners to explore the transformative i...
Pierre Fabre Laboratories receive positive CHMP opinion for OBGEMSA™(vibegron) in overactive bladder syndrome
CASTRES, France, April 26, 2024 /PRNewswire/ -- Pierre Fabre Laboratories announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending approval of OBGEMSA™ (vibegron under the international non-propriet...
Novel T-cell engager, CDH17 X CD3 cabotamig (ARB202) continues to explore dosing in patients with advanced gastrointestinal cancers
* The second DMC review recommends the continuation of dose escalation in the clinical trial as per protocol. * The interim safety assessment was based on the review of safety data from 18 metastatic gastrointestinal cancer patients, including patients that have tolerated multiple doses of ca...
Fosun Pharma's Self-developed Artemisinin Medicines Inject New Impetus to Malaria Prevention and Treatment in Africa
SHANGHAI, April 26, 2024 /PRNewswire/ -- World Malaria Day is marked each year onApril 25. World Health Organization (WHO) gave as the theme for World Malaria Day 2024Accelerating the fight against malaria for a more equitable world. WHO stated that malaria not only continues to directly endanger...
2024 ASCO | Mabwell to Present Clinical Data of 9MW2821 in Multiple Advanced Solid Tumor
SHANGHAI, April 25, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with an entire industry chain, announced that the Phase I/II clinical study results of the novel Nectin-4-targeting ADC 9MW2821 for multiple advanced solid tumors will be presented in an ...
Antengene Announces One Oral and Three Poster Presentations at ASCO 2024
* Oral session:ATG-008 (mTORC1/2 Inhibitor) combined with PD-1 antibody in Phase II studies for cervical cancer * Three Poster presentations: Phase I/II studies for ATG-031 (anti-CD24 monoclonal antibody) and ATG-022 (Claudin 18.2 antibody-drug conjugate) and selinexor(XPO1 Inhibitor) SHANGHA...
Onconic Therapeutics Receives MFDS Approval for JAQBO, a New Treatment for Gastroesophageal Reflux Disease (GERD)
SEOUL, South Korea, April 25, 2024 /PRNewswire/ -- Onconic Therapeutics, a leading biotechnology company in Korea, today announced that the Ministry of Food and Drug Safety (MFDS) of Korea has approved JAQBO (zastaprazan citrate) for the treatment of erosive gastroesophageal reflux disease (GERD)...
Week's Top Stories
Most Reposted
VentureBlick Launches 'Discovery': The First Global Networking Platform for Healthcare Innovation
[Picked up by 321 media titles]
2024-05-08 14:37OPTIX raises US$15 million fundraising to date to solve the bottleneck of XR optics technology
[Picked up by 312 media titles]
2024-05-08 08:00IN2MF in Kuala Lumpur Presented by Bank Indonesia
[Picked up by 299 media titles]
2024-05-08 16:44Thousands set to gather in Singapore for Rotary's international convention, the Garden City's largest association event post-pandemic
[Picked up by 289 media titles]
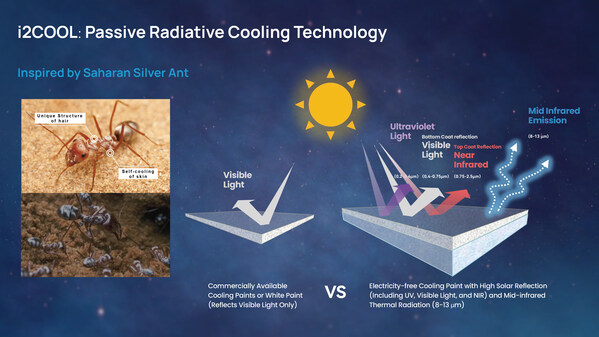
2024-05-09 11:00i2Cool Attracts Close to HKD100 Million in Series A Financing to Propel the Development of Green and Energy-Efficient Radiant Cooling Solutions
[Picked up by 283 media titles]
2024-05-09 10:50