Health Care/Hospital
Ractigen Announces First Patient Dosed in the Phase I Clinical Trial of RAG-01 for NMIBC
JIANGSU, China, April 3, 2024 /PRNewswire/ -- Ractigen Therapeutics, a pioneer in small activating RNA (saRNA) therapeutics, achieved a major milestone today with the first patient dosed in its First-in-human phase I clinical trial for RAG-01 conducted in collaboration with GenesisCare,Australia'...
Ascletis Announces Strategic Decisions on FXR agonist ASC42
HANGZHOU, China and SHAOXING, China, April 3, 2024 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672, "Ascletis") announces today the strategic decisions on farnesoid X receptor (FXR) agonist ASC42. After thorough analysis of the Phase II trial data of ASC42 for primary biliary cholangitis (PBC) (...
Nagase Viita Receives Highest "Platinum" Rating for Sustainability from EcoVadis
OKAYAMA, Japan, April 3, 2024 /PRNewswire/ -- Nagase Viita Co., Ltd., a NAGASE Group member headquartered in Okayama,Japan, has received the highest "Platinum" rating in a sustainability survey conducted by EcoVadis SAS ofFrance , one of the world's most trusted providers of business sustainabilit...
I-Mab Announces Closing of the Divestiture of Business Operations in China
ROCKVILLE, Md., April 2, 2024 /PRNewswire/ -- I-Mab (the "Company") (NASDAQ: IMAB) a U.S.-based, global biotech company, exclusively focused on the development and potential commercialization of highly differentiated immunotherapies for the treatment of cancer, today announced that all condition...
GC Cell to Present Multiple Posters at the American Association for Cancer Research (AACR) Annual Meeting 2024
YONGIN, South Korea, April 2, 2024 /PRNewswire/ -- GC Cell
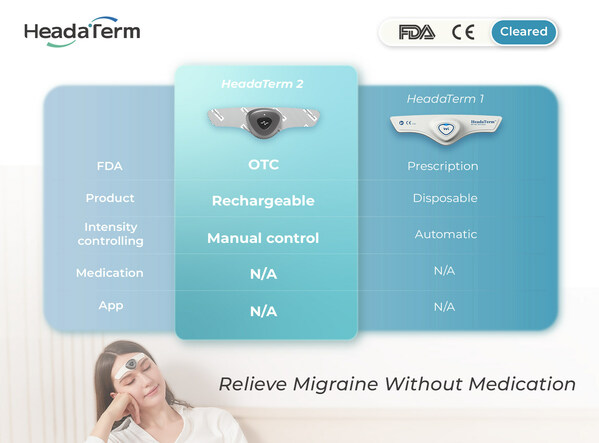
HeadaTerm 2: The Most Affordable FDA Cleared Innovative OTC Anti-Migraine Device
VANCOUVER, BC, April 2, 2024 /PRNewswire/ -- WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S. U...
China Medical University Hospital Develops "AISIA" to Diagnose Acute Ischemic Stroke in 90 Seconds to Assist Doctors in Making Medical Decisions
TAICHUNG, Taiwan , April 2, 2024 /PRNewswire/ -- The Neurology Department of China Medical University Hospital (CMUH,Taiwan), teaming up with AI Center at CMUH, has established the AISIA (Artificial Intelligence for Stroke Image Analysis) platform, which contains the "NCCT-based Ischemic Stroke D...
Innovating "Treadmill Exercise Test AI-Assisted Interpretation System," CMUH(Taiwan) Timely Saves More Patients with Severe Myocardial Infarction
TAICHUNG, Taiwan, April 2, 2024 /PRNewswire/ -- The treadmill exercise test (TET) is an important tool for diagnosing coronary artery disease (CAD), but limited by time spent for the manual interpretation of a dozen charts and the challenges in identifying minor deviations. Traditionally, manual ...
China Medical University Hospital, Collaborated with Microsoft Taiwan, Launched GenAI: gHi System
gHi system is the world's first GenAI medical record system in Mandarin and reduce time spent by 75% TAICHUNG, Taiwan, April 2, 2024 /PRNewswire/ -- China Medical University Hospital (CMUH) and Microsoft Taiwan announced the Generative Healthcare Intelligent (gHi) System, which is being applied...
First Patient Dosed in Clinical Trial of YOLT-101 for the Treatment of FH
SHANGHAI, April 2, 2024 /PRNewswire/ -- YolTech Therapeutics announced that the first patient has been dosed with YOLT-101, the company'sin vivo genome editing candidate being developed as a single dose, potentially curative therapy for Familial Hypercholesterolemia(FH), marking the commencement ...
Gushengtang Releases 2023 Annual Results: Sustained Growth with Robust Profit Increase
GUANGZHOU, China, April 2, 2024 /PRNewswire/ -- On March 27th, Gushengtang announced its financial results for 2023. During the year, the company's revenue reached2.323 billion yuan, marking a 43% year-over-year increase; the adjusted net profit was305 million yuan, increased by 53.6% compared to...
23WELL, THE health supplement brand on the watch
LOS ANGELES, April 1, 2024 /PRNewswire/ -- Just earlier last month, the highly anticipated Natural Products Expo West 2024 concluded successfully inAnaheim, CA. As one of the world's largest leading health and nutrition exhibition, it hosted more than 65,000 registered attendees and 3,000+ exhibi...
Lunit to Showcase 7 Studies at AACR 2024: Unveiling AI Innovations in HER2 Expression-Mutation Analysis and CNTN4 Biomarker Identification
- Lunit's AACR 2024 presentations to spotlight HER2 mutation insights and CNTN4's role in immunotherapy success, pioneering next-gen personalized treatment approaches, supported by the AI-powered Lunit SCOPE suite SEOUL, South Korea, April 1, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading...
Aculys Pharma Announces Appointment of Hidemasa Tanigaki as New CEO
~Hidemasa's appointment bolsters Aculys' position as a leader in the field of CNS inJapan and Asia~ TOKYO, April 1, 2024 /PRNewswire/ -- Aculys Pharma, Inc. ("Aculys"), a clinical-stage biopharmaceutical company focused on commercializing innovative treatments for neurological conditions, today ...
OFFICIAL LAUNCH OF THE NATIONAL UNIVERSITY SPINE INSTITUTE (NUSI): LEADING SPINE CARE INTO THE FUTURE
Public symposium by NUSI marks the end of a series of spine-related events that provide doctors with platform for professional development and networking SINGAPORE, March 30, 2024 /PRNewswire/ -- The National University Spine Institute (NUSI) is a new dedicated specialist spine institute under th...
Baird Medical Granted New Class III Certificate by China's NMPA for Ceramic Thyroid Ablation Needle
FRISCO, Texas, March 29, 2024 /PRNewswire/ -- Baird Medical Devices, Inc. ("Baird Medical" or the "Company"), a leading microwave ablation ("MWA") medical device developer and provider inChina and the United States, today announced it has been granted a new Class III certificate by the National ...
LISCure Biosciences receives U.S. FDA Fast Track designation for LB-P8 for the treatment of primary sclerosing cholangitis (PSC)
* Phase 2 study is underway and LB-P8 is the only live biotherapeutic product currently reported to be in clinical development for the treatment of PSC * FDA's Fast Track designation for LB-P8 underlines the urgent need for treatment options to fulfill the unmet medical needs of people affecte...
73% CNS ORR! FDA Granted ODD to Utidelone Injectable (UTD1) from Biostar Pharma for the Treatment of Breast Cancer Brain Metastasis
SAN FRANCISCO, March 29, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the U.S. subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the development and commercialization of innovative oncology drugs, announced today that their cor...
Alebund's Innovative Investigational Drug AP303 Receives FDA Orphan Drug Designation (ODD) for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
SHANGHAI, March 29, 2024 /PRNewswire/ -- Alebund Pharmaceuticals
IASO Bio Announces NMPA's IND Approval for Equecabtagene Autoleucel in Second- and Third-Line Treatment of Multiple Myeloma
SHANGHAI and NANJING, China and SAN JOSE, Calif., March 29, 2024 /PRNewswire/ -- IASO Bio, a biopharmaceutical company engaged in discovering, developing, manufacturing and marketing innovative cell therapies and antibody products, today announced thatChina National Medical Products Administratio...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 272 media titles]
2024-04-24 17:09