SEOUL, South Korea, May 29, 2019 /PRNewswire/ -- JLK Inspection, a company specializing in world-class AI-based medical imaging solutions and a company of the Born2Global Centre, has developed JPC-01K, an AI-based prostate MR image analysis solution, and JBS-01K, which is used for quantitative analysis of brain MR images, and recently obtained European CE certification for such products, in accordance with the European Medical Device Directive (MDD).
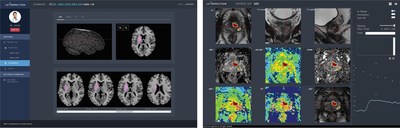
“JBS-01K” AI-based automatic analysis of stroke using MR images (left) and “JPC-01K” AI-based automatic analysis of prostate using MR Images (right)
The CE MDD, a certification process that assesses quality, efficacy, effectiveness, durability, and safety, as suggested by the European Union, is essential for companies seeking to enter the European market and can be obtained only if highly strict requirements are met.
A representative of JLK Inspection explained, "It is noteworthy that our solutions have been acknowledged as products that meet world-class quality standards."
Having satisfied an international quality standard that encompasses all of Europe, JLK Inspection is now planning to enter the global market as soon as possible.
Meanwhile, last year, JLK Inspection released AIHuB, an all-in-one medical platform comprising 37 medical solutions, including JPC-01K and JBS-01K.
AIHuB is capable of detecting and monitoring 37 medical conditions in 14 different regions of the body. It can also conduct analysis based on multimodal images, including MRI, CT, X-ray, and mammogram images, using an AI-enabled technology that encompasses a wide variety of techniques for diagnosing illnesses, such as stroke, Alzheimer's, and cancer.
Having released AIHuB, JLK Inspection's action plan to enter the global market includes active participation in several leading international exhibitions and conferences, including RSNA (Radiological Society of North America) 2019, ECR (European Congress of Radiology) 2019, and CES (Consumer Electronics Show) 2020.
Now that it has received CE certification, JLK Inspection is expected to make preparations to engage in aggressive overseas marketing. A representative of the company stated, "Starting with CE certification, we are planning to obtain FDA approval and expand our market to the United States in the foreseeable future."
Media Contact
JLK Inspection: MG@jlk-inspection.com
Born2Global Centre: jlee@born2global.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/ce-certification-for-ai-based-medical-solutions-of-jlk-inspection-ai-based-brain-image-analysis-jbs-01k-and-ai-based-prostate-image-analysis-jpc-01k-solutions-300858180.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/ce-certification-for-ai-based-medical-solutions-of-jlk-inspection-ai-based-brain-image-analysis-jbs-01k-and-ai-based-prostate-image-analysis-jpc-01k-solutions-300858180.html