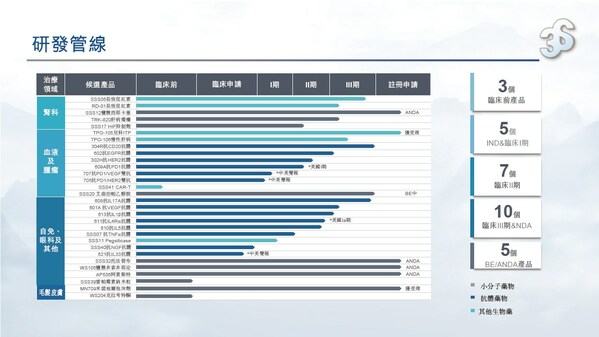
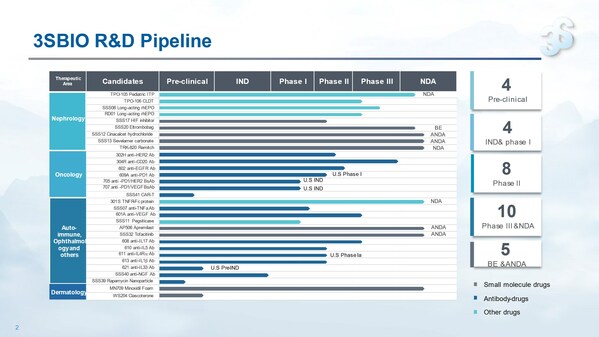
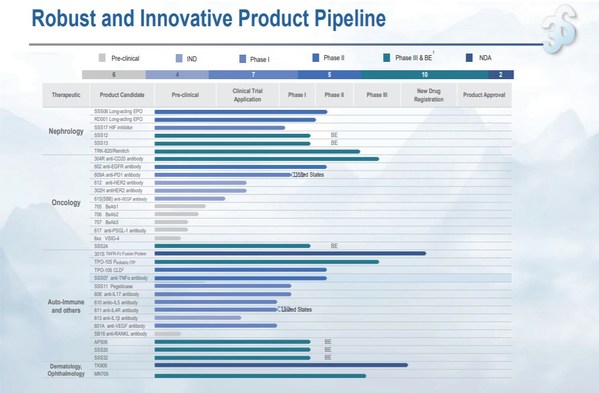
3SBio announces 2023 interim results, with revenue growing over 20% year on year and pipeline value constantly enhanced
HONG KONG, Aug. 25, 2023 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today released its 2023 interim results. Revenue in the first half of 2023 reached approximatelyRMB3,783.8 million, up 22.3% on a yearly basis. Gross profit was approximatelyRMB3,201.6 million, up ...
3SBio announces 2022 annual results, with revenue rising 7.5% year on year and normalized net profit attributable to parents jumping 25.2%
HONG KONG, March 22, 2023 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today announced its 2022 annual results. In 2022, 3SBio's core biopharmaceutical products posted robust growth, hair health business achieved blistering performance, and contract development and ...
HanBio Therapeutics Announces the Completion of a US$40 Million Series A Financing Led by OrbiMed and Hankang Capital
SHANGHAI, Jan. 28, 2022 /PRNewswire/ -- HanBio Therapeutics ("HanBio"), a biotechnology company committed to researching and developing next generation transformative medicines, today announced that it has completed aUS$40 million Series A financing to advance the preclinical and clinical develop...
3SBio announces 2021 interim results, with strong growth in core businesses and significant progress in clinical development
HONG KONG, Aug. 26, 2021 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today unveiled its 2021 interim results. 3SBio maintained strong performance in core businesses, with core products occupying a leading market share. Meanwhile, the Company continuously increased ...
3SBio announces 2020 annual results, tapping integrated platform advantages for steady growth
SHANGHAI, March 30, 2021 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today unveiled its 2020 full-year results. The Company maintained stable profitability despite the huge challenge amid the COVID-19 pandemic, while ushering in new milestones. It once again demonst...
3SBio Inc. received milestone payment of USD 4 million upon commencement of phase III clinical program of SEL-212
SHENYANG, China, Nov. 23, 2020 /PRNewswire/ -- Chinese leading biopharmaceutical company, 3SBio Inc.(01530.HK, the "Company"), announced today that Selecta Biosciences, Inc. (Nasdaq: SELB), the Company's partner, has commenced the phase III clinical program of a combination therapy involving SEL...
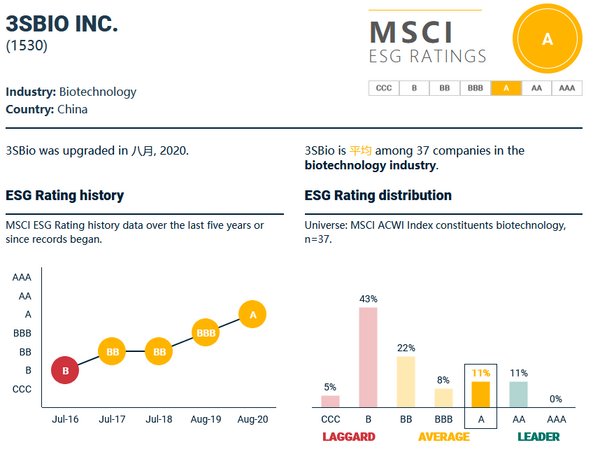
3SBio MSCI ESG Rating Upgraded to A, Ranking at the Forefront of the Global Biotechnology Industry
SHANGHAI, Sept. 2, 2020 /PRNewswire/ -- According to Morgan Stanley Capital International's (MSCI) latest set of ESG ratings forAugust 26th 2020, 3SBio has been upgraded to A status, surpassing that of 78% of companies in the global biotechnology industry. MSCI ESG ratings focus on ESG issues th...
3SBio Unveils 2019 Annual Results: Revenue Rises by 16.0%, Normalized Net Profit attributable to owners of the parent Jumps by19.4%, R&D Expenses Soar 45.2%
HONGKONG, March 31, 2020 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today released its 2019 annual results, showing that the Company maintained steady growth, with core products continuously leading the market and more products being included into drug reimbursemen...
3SBio Selects Verseau's PSGL-1-targeted Antibody VTX-0811 as First Partnered Macrophage Checkpoint Modulator in Immuno-Oncology Collaboration
SHENYANG, China and BEDFORD Mass., Nov. 17, 2019 /PRNewswire/ -- 3SBio Inc.
Submission and acceptance of application for the production of new drug for Yisaipu(R)aqueous injection solution
SHANGHAI, July 29, 2019 /PRNewswire/ -- 3SBio Inc. (01530.HK), a leading biopharmaceutical company in the PRC, announces today that an application for the production of new drug has been submitted to the National Medical Products Administration for Yisaipu® pre-filled aqueous injection solution (...
3SBio Unveils 2018 Annual Results: Revenue Soars by 22.7%, Normalized Net Profit Soars by 29.0%, R&D Expenses Jumps Over 40%
HONG KONG, March 21, 2019 /PRNewswire/ -- Chinese leading biotechnology company 3SBio (01530.HK) today released its 2018 annual results, showing that the Company has been posting dramatic business growth, and has been gearing up R&D layout and investment in innovative drugs. Financial highlights...
3SBio and TLC Form Exclusive Partnership to Commercialize Two NanoX(TM) Products in Mainland China
SHENYANG, China, March 4, 2019 /PRNewswire/ -- 3SBio Inc. ("3SBio") (HKEX: 1530) and Taiwan Liposome Company, Ltd. ("TLC") (Nasdaq: TLC, TWO: 4152) today announced an exclusive partnership to commercialize in mainlandChina two liposomal products utilizing TLC's proprietary NanoX™ technology platf...
3SBio and Verseau Establish Global Clinical Development Collaboration for First-in-class Immuno-Oncology Therapies
SHENYANG, China and BOSTON, Feb. 11, 2019 /PRNewswire/ -- 3SBio Inc. ("3SBio") (HKEX:1530) and Verseau Therapeutics, Inc. ("Verseau") announced today a partnership agreement focused on the development and commercialization of novel monoclonal antibodies in the field of immuno-oncology for a broad...
3SBio and Verseau Establish Global Clinical Development Collaboration for First-in-class Immuno-Oncology Therapies
SHENYANG, China and BOSTON, Feb. 11, 2019 /PRNewswire/ -- 3SBio Inc. ("3SBio") (HKEX:1530) and Verseau Therapeutics, Inc. ("Verseau") announced today a partnership agreement focused on the development and commercialization of novel monoclonal antibodies in the field of immuno-oncology for a broad...
3SBio Unveils 2017 Annual Results: Revenue Soars by 33.5%
HONG KONG, March 26, 2018 /PRNewswire/ -- Leading Chinese biotechnology company 3SBio(01530.HK) today released its 2017 annual results, showing that the company has been dramatically growing with important progress made in all businesses. Looking to the future, the company intends to reinforce it...
China FDA Approves New Once-Weekly Bydureon(R) to Improve Glycemic Control in Patients with Type-2 Diabetes
SHANGHAI, Jan. 4, 2018 /PRNewswire/ -- 3Sbio Inc. (01530.HK) announced today thatChina's first Glucagon-like peptide-1 (GLP-1) receptor agonist weekly preparation Bydureon® (generic name Exenatide Microsphere for injection) has been formally approved by China Food and Drug Administration (CFDA), ...
3SBio Accelerates Expansion of its Global Biologics Platform by Acquiring the Canadian Biomanufacturing Business of Therapure
SHENYANG China, Sept. 3, 2017 /PRNewswire/ -- 3SBio Inc. (01530.HK, the "Group"), a leading Chinese biopharmaceutical company, is pleased to announce today that it has entered into a Shareholder Agreement with CPE Funds, pursuant to which a Joint Venture (the "JV") shall be established. The Group...
3SBio Inc. Appoints Dr. Zhenping Zhu as President of Research & Development and Chief Scientific Officer
SHENYANG, China, Jan. 9, 2017 /PRNewswire/ -- 3SBio Inc., (HKEX:1530) ("3SBio" and together with its subsidiaries the "3SBio Group") is pleased to announce thatZhenping Zhu, MD, PhD, has joined 3SBio as its new President of Research & Development and Chief Scientific Officer. "We are pleased to ...
3SBio Inc. Selected as a Constituent of the MSCI China Index
SHENYANG, China, May 13, 2016 /PRNewswire/ -- 3SBio Inc., (HKEX:1530) ("3SBio") today announced that according to an announcement posted on the MSCI Inc. website onMay 12, 2016, 3SBio will be added as a constituent to the MSCI China Index after the market closes onMay 31, 2016. "We are pleased ...
3SBio's PEG-irinotecan Wins IND Approval From China FDA
SHENYANG, China, March 7, 2016 /PRNewswire/ -- 3SBio Inc., ("3SBio") a leading biotechnology company based inChina focusing on researching, developing, manufacturing and marketing biopharmaceutical products, announced today that it has received an Investigational New Drug ("IND") approval letter ...