Ascletis Announces IND Approval of Viral Polymerase Inhibitor ASC10 for Monkeypox Indication by U.S. FDA
--Based on available data, ASC10 at the dosage of 800 mg twice daily was approved by the U.S. Food and Drug Administration (FDA) to conduct a Phase Ib study in patients with monkeypox virus disease --Preclinical studies show that ASC10-A, active metabolite of double prodrug ASC10, has potent ant...
Ascletis Announces Poster Presentation of Phase I, Single-Dose Study of ASC43F for NASH at AASLD Annual Meeting 2022
HANGZHOU and SHAOXING, China, Nov. 7, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672, "Ascletis") announces today that the abstract of a Phase I, Single-Dose Study of ASC43F for non-alcoholic steatohepatitis (NASH) has been reported at The Liver Meeting® 2022 of the American Association for...
Ascletis Announces U.S. IND Filing of Oral 3CLpro Inhibitor ASC11 for COVID-19
-- ASC11 is an in-house discovered oral small molecule drug candidate, targeting 3-chymotrypsin like protease (3CLpro) -- In antiviral cellular assays with infectious SARS-CoV-2, ASC11 demonstrated higher potency against SARS-CoV-2 than other 3CLpro inhibitors including Nirmatrelvir, S-217622, P...
Ascletis Announces U.S. IND Filing of Oral Antiviral ASC10 for Monkeypox Indication
--ASC10 has two indications: monkeypox and SARS-CoV-2 virus infections. The Investigational New Drug (IND) application of the latter was approved by the U.S. Food and Drug Administration (FDA) inAugust 2022 and Phase Ib clinical trial in COVID-19 patients is underway in the U.S. --Preclinical st...
Ascletis Announces Dosing of 24 Healthy Subjects of the First 3 Cohorts in Multiple-Dose Escalation Phase I Clinical Trial of Oral RdRp Inhibitor ASC10 for COVID-19
--The multiple-dose escalation Phase I clinical trial will enroll 72 healthy subjects including 60 subjects in 6 dose escalation cohorts and 12 subjects in food effect trial. The enrollment is expected to be completed in the fourth quarter of 2022 --ASC10 is an oral double prodrug. After oral ...
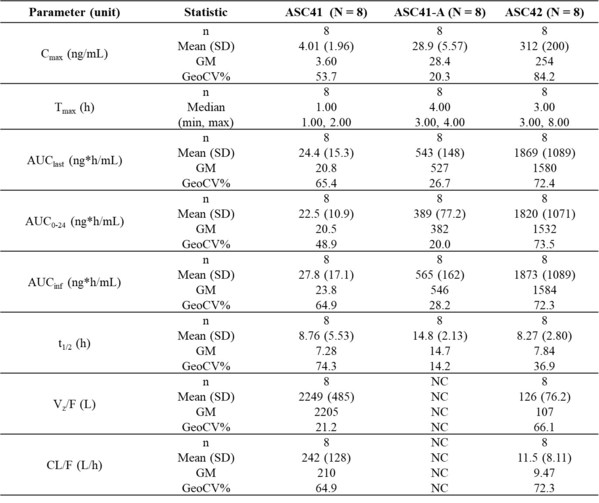
Ascletis Announces Dosing of the First Patient in Phase II Clinical Trial of THRβ Agonist ASC41 for 52-Week Treatment of Liver Biopsy-Proven NASH
-- ASC41 is ranking first in China and third in the world in terms of clinical progress as a thyroid hormone receptor β (THRβ) agonist drug candidate for non-alcoholic steatohepatitis (NASH). ASC41 Phase II clinical trial is currently the most advanced 52-week Phase II clinical trial which is in...
Ascletis Announces Dosing of the First Patient in the Phase IIb Expansion Cohort of ASC22 (Envafolimab) for Chronic Hepatitis B Functional Cure
-- After the pre-Phase III clinical trial meeting with Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) in June 2022, the pathway to the registration, including patient population, dose, treatment duration, etc. of ASC22 (Envafolimab) for functional cure of...
Shanghai Public Health Clinical Center Completed Patient Enrollment in Clinical Study of PD-L1 Antibody ASC22 (Envafolimab) in Combination with Chidamide for Functional Cure of HIV Infection
HANGZHOU and SHAOXING, China, Sept. 15, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that the clinical study of PD-L1 antibody ASC22 (Envafolimab) in combination with Chidamide for functional cure of human immunodeficiency virus (HIV) infection has completed ...
Ascletis Announces IND Approval of Oral RdRp Inhibitor ASC10 for COVID-19 by China NMPA
-- Ascletis is China's first biotech company which has obtained IND approvals of an oral RdRp inhibitor from both China NMPA and the U.S. FDA -- Ascletis has filed multiple patent applications for ASC10 and its use globally. Compared with molnupiravir, ASC10 has a new and differentiated chemical...
Gannex Announces First Subject Dosed in the U.S. Drug-Drug Interaction Study of FXR Agonist ASC42 for Treatment of Primary Biliary Cholangitis
-- Enrollment of the total 12 subjects is expected to be completed in August 2022 -- The DDI study on ASC42 in the U.S. is expected to be completed by the beginning of the fourth quarter 2022 -- This DDI study and ongoing Phase II clinical trial in PBC patients in China will provide more eviden...
Ascletis Announces First Patient Dosed in the U.S. Phase I Clinical Trial of Oral PD-L1 Small Molecule Inhibitor Prodrug ASC61 for Treatment of Advanced Solid Tumors
--ASC61 is an in-house developed oral PD-L1 small molecule inhibitor prodrug that showed significant antitumor efficacy in preclinical studies as a single agent in multiple animal models --ASC61-A treatment induced secretion of IFNγ in a concentration dependent manner with an EC50 of 2.86 nM. Ma...
Ascletis Announces IND Filing of Oral RdRp Inhibitor ASC10 for COVID-19 Accepted by China NMPA
HANGZHOU, China and SHAOXING, China, Aug. 4, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that the Investigational New Drug (IND) application of ASC10, an oral inhibitor drug candidate targeting RNA-dependent RNA polymerase (RdRp) for COVID-19, has been accep...
Ascletis Announces FDA Clearance of Oral RdRp Inhibitor ASC10 to Conduct a Randomized, Placebo Controlled Phase Ib Study in Mild-to-Moderate COVID-19 Patients
-- ASC10 is an oral double prodrug that is rapidly and completely converted in vivo into the active metabolite ASC10-A, which is the same active metabolite of molnupiravir -- Ascletis has filed multiple patent applications for ASC10 and its use globally. Compared with molnupiravir, ASC10 has a n...
Ascletis Announces U.S. IND Filing of Oral RdRp Inhibitor Drug Candidate ASC10 for COVID-19
-- ASC10 is an oral small molecule drug candidate, which is in-house discovered and developed, and Ascletis retains full global rights for its development and commercialization --This IND filing of ASC10 in the U.S. will accelerate Ascletis' global multi-center clinical studies on ASC10 and bett...
Shanghai Public Health Clinical Center Completed the First Patient Dosing in Clinical Study of PD-L1 Antibody ASC22 in Combination with Chidamide for Functional Cure of HIV Infection
HANGZHOU and SHAOXING, China, July 4, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that the clinical study of PD-L1 antibody ASC22 in combination with Chidamide for functional cure of human immunodeficiency virus (HIV) infection, which was initiated by Shangh...
Ascletis Announced First Subject Dosed in the Phase II Clinical Trial of ASC22 (Envafolimab) for Immune Restoration/ Functional Cure of HIV-1 Infection
-- The Phase II clinical study in China is currently expected to be completed in early 2023 with an expected enrollment of 30 subjects HANGZHOU, China and SHAOXING, China, June 28, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that the first subject has been ...
Ascletis' Subcutaneous PD-L1 Antibody ASC22 Demonstrated Potential of Functional Cure of Chronic Hepatitis B as 42.9% of Patients with Baseline HBsAg≤100 IU/mL Obtained Sustained HBsAg Loss
--Ascletis presented Phase IIb clinical trial results of subcutaneous PD-L1 antibody ASC22 for functional cure of CHB at oral session of EASL ILC 2022 on June 25, 2022, Beijing Time --The Phase IIb clinical trial results further demonstrated the potential of ASC22+NAs treatment as a functional cu...
Ascletis Announces Oral Presentation on Updates from Phase IIb Clinical Trial of ASC22, a Subcutaneous PD-L1 Antibody for Functional Cure of Chronic Hepatitis B at EASL ILC 2022
- The updates of ASC22 Phase IIb study will be presented at the oral session during the International Liver Congress™ 2022 onSaturday, June 25, 2022 at 14:15 , Beijing Time (Abstract Number: OS091) HANGZHOU, China and SHAOXING, China, June 16, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672...
Ascletis Announces Appointment of Mr. John P. Gargiulo, Former North America President and CEO of Daiichi Sankyo, as Chief Business Officer
HANGZHOU, China and SHAOXING, China, June 13, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis"), today announces the expansion of management team with appointment of Mr.John P. Gargiulo, former North America President and CEO of Daiichi Sankyo, as Chief Business Officer. Mr.John ...
Gannex Announces U.S. FDA Clearance of Clinical Trial on FXR Agonist ASC42 for Treatment of Primary Biliary Cholangitis
-- ASC42 has completed Phase I trials in the U.S. and China. This approval from U.S. FDA enables Gannex to complete a critical drug-drug interaction (DDI) study to support upcoming Phase III trials inChina, the U.S. and European Union -- Gannex expects to complete this DDI study at the beginning ...