Ascletis Announces Notice of Issuance of the U.S. Patent for Oral Viral Polymerase Inhibitor ASC10 and Its Derivatives
--The issue date of the patent will be January 3, 2023 by the United States Patent and Trademark Office (USPTO). The patent number is 11,541,071 -- The issued patent covers ASC10 and its derivatives, and their uses to treat virus infections including SARS-CoV-2, monkeypox virus and respiratory s...
Ascletis Announces Positive Phase I Clinical Results of Oral RdRp Inhibitor ASC10 for COVID-19
--The exposure of active drug ASC10-A after twice daily dosing 800 mg double prodrug ASC10 in Chinese subjects was 94% of that after twice daily dosing 800 mg single prodrug molnupiravir in Japanese subjects --Based on Ascletis' Phase I results of ASC10 and molnupiravir's clinical efficacy data ...
Ascletis Announces IND Approval of Oral 3CLpro Inhibitor ASC11 for COVID-19 by China NMPA
--The objective of Phase I clinical trial is to identify a safe and efficacious dose for the pivotal Phase II/III in COVID-19 patients. Phase I clinical trial is expected to be completed within the first quarter of 2023 --Phase I clinical trial will also determine if ASC11 needs to be boosted by ...
Ascletis Announces Completion of 180 Patient Enrollment for Phase II Clinical Trial of FASN Inhibitor ASC40 for Acne
--To date, approximately 50% enrolled patients have completed 12-week treatment and all enrolled patients are expected to complete 12-week treatment by the end ofFebruary 2023 -- Clinical efficacy observed in patients who have completed 12-week treatment of ASC40 or placebo was comparable t...
Ascletis Announces Completion of 180 Patient Enrollment for Phase II Clinical Trial of FASN Inhibitor ASC40 for Acne
--To date, approximately 50% enrolled patients have completed 12-week treatment and all enrolled patients are expected to complete 12-week treatment by the end ofFebruary 2023 -- Clinical efficacy observed in patients who have completed 12-week treatment of ASC40 or placebo was comparable t...
Ascletis Announces IND Filing of Oral 3CLpro Inhibitor ASC11 for COVID-19 Accepted by China NMPA
HANGZHOU, China and SHAOXING, China, Nov. 29, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") announces today that the Investigational New Drug (IND) application of ASC11, an oral inhibitor drug candidate targeting 3-chymotrypsin like protease (3CLpro) for COVID-19, has been ac...
Ascletis Announces IND Approval of Oral 3CLpro Inhibitor ASC11 for COVID-19 by U.S. FDA
--The Phase I clinical trial will consist of 3 cohorts in healthy subjects, including single- and multiple-dose escalation studies and food effect study. The objective of Phase I trial is to find a right dose to move into the pivotal Phase II/III in COVID-19 patients --In antiviral cellular assa...
Ascletis Announces IND Approval of Oral PD-L1 Small Molecule Inhibitor ASC61 for Treatment of Advanced Solid Tumors by China NMPA
--While the ASC61 Phase I study is ongoing in the U.S., the Investigational New Drug (IND) approval inChina will accelerate the global development of ASC61, an in-house developed oral PD-L1 small molecule inhibitor HANGZHOU and SHAOXING, China, Nov. 17, 2022 /PRNewswire/ -- Ascletis Pharma Inc. ...
Ascletis Announces IND Approval of Viral Polymerase Inhibitor ASC10 for Monkeypox Indication by U.S. FDA
--Based on available data, ASC10 at the dosage of 800 mg twice daily was approved by the U.S. Food and Drug Administration (FDA) to conduct a Phase Ib study in patients with monkeypox virus disease --Preclinical studies show that ASC10-A, active metabolite of double prodrug ASC10, has potent ant...
Ascletis Announces Poster Presentation of Phase I, Single-Dose Study of ASC43F for NASH at AASLD Annual Meeting 2022
HANGZHOU and SHAOXING, China, Nov. 7, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672, "Ascletis") announces today that the abstract of a Phase I, Single-Dose Study of ASC43F for non-alcoholic steatohepatitis (NASH) has been reported at The Liver Meeting® 2022 of the American Association for...
Ascletis Announces U.S. IND Filing of Oral 3CLpro Inhibitor ASC11 for COVID-19
-- ASC11 is an in-house discovered oral small molecule drug candidate, targeting 3-chymotrypsin like protease (3CLpro) -- In antiviral cellular assays with infectious SARS-CoV-2, ASC11 demonstrated higher potency against SARS-CoV-2 than other 3CLpro inhibitors including Nirmatrelvir, S-217622, P...
Ascletis Announces U.S. IND Filing of Oral Antiviral ASC10 for Monkeypox Indication
--ASC10 has two indications: monkeypox and SARS-CoV-2 virus infections. The Investigational New Drug (IND) application of the latter was approved by the U.S. Food and Drug Administration (FDA) inAugust 2022 and Phase Ib clinical trial in COVID-19 patients is underway in the U.S. --Preclinical st...
Ascletis Announces Dosing of 24 Healthy Subjects of the First 3 Cohorts in Multiple-Dose Escalation Phase I Clinical Trial of Oral RdRp Inhibitor ASC10 for COVID-19
--The multiple-dose escalation Phase I clinical trial will enroll 72 healthy subjects including 60 subjects in 6 dose escalation cohorts and 12 subjects in food effect trial. The enrollment is expected to be completed in the fourth quarter of 2022 --ASC10 is an oral double prodrug. After oral ...
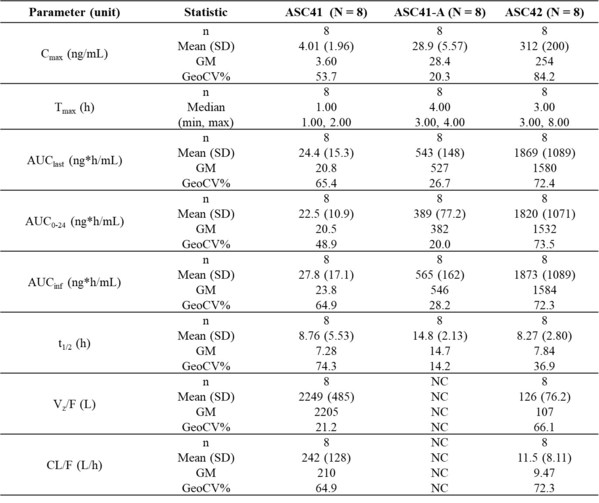
Ascletis Announces Dosing of the First Patient in Phase II Clinical Trial of THRβ Agonist ASC41 for 52-Week Treatment of Liver Biopsy-Proven NASH
-- ASC41 is ranking first in China and third in the world in terms of clinical progress as a thyroid hormone receptor β (THRβ) agonist drug candidate for non-alcoholic steatohepatitis (NASH). ASC41 Phase II clinical trial is currently the most advanced 52-week Phase II clinical trial which is in...
Ascletis Announces Dosing of the First Patient in the Phase IIb Expansion Cohort of ASC22 (Envafolimab) for Chronic Hepatitis B Functional Cure
-- After the pre-Phase III clinical trial meeting with Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) in June 2022, the pathway to the registration, including patient population, dose, treatment duration, etc. of ASC22 (Envafolimab) for functional cure of...
Shanghai Public Health Clinical Center Completed Patient Enrollment in Clinical Study of PD-L1 Antibody ASC22 (Envafolimab) in Combination with Chidamide for Functional Cure of HIV Infection
HANGZHOU and SHAOXING, China, Sept. 15, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that the clinical study of PD-L1 antibody ASC22 (Envafolimab) in combination with Chidamide for functional cure of human immunodeficiency virus (HIV) infection has completed ...
Ascletis Announces IND Approval of Oral RdRp Inhibitor ASC10 for COVID-19 by China NMPA
-- Ascletis is China's first biotech company which has obtained IND approvals of an oral RdRp inhibitor from both China NMPA and the U.S. FDA -- Ascletis has filed multiple patent applications for ASC10 and its use globally. Compared with molnupiravir, ASC10 has a new and differentiated chemical...
Gannex Announces First Subject Dosed in the U.S. Drug-Drug Interaction Study of FXR Agonist ASC42 for Treatment of Primary Biliary Cholangitis
-- Enrollment of the total 12 subjects is expected to be completed in August 2022 -- The DDI study on ASC42 in the U.S. is expected to be completed by the beginning of the fourth quarter 2022 -- This DDI study and ongoing Phase II clinical trial in PBC patients in China will provide more eviden...
Ascletis Announces First Patient Dosed in the U.S. Phase I Clinical Trial of Oral PD-L1 Small Molecule Inhibitor Prodrug ASC61 for Treatment of Advanced Solid Tumors
--ASC61 is an in-house developed oral PD-L1 small molecule inhibitor prodrug that showed significant antitumor efficacy in preclinical studies as a single agent in multiple animal models --ASC61-A treatment induced secretion of IFNγ in a concentration dependent manner with an EC50 of 2.86 nM. Ma...
Ascletis Announces IND Filing of Oral RdRp Inhibitor ASC10 for COVID-19 Accepted by China NMPA
HANGZHOU, China and SHAOXING, China, Aug. 4, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that the Investigational New Drug (IND) application of ASC10, an oral inhibitor drug candidate targeting RNA-dependent RNA polymerase (RdRp) for COVID-19, has been accep...