Brii Biosciences Announces Publication of Phase 2 ENSURE Study Results in Nature Medicine
* Findings provide scientific insights into the contribution of small
interfering RNA (siRNA) and therapeutic vaccination towards the HBV functional
cure strategy DURHAM, N.C. and BEIJING, Nov. 7, 2025 /PRNewswire/ -- Brii
Biosciences Limited
Brii Biosciences Provides Corporate Updates and Reports 2025 Interim Financial Results
Multiple Ongoing Phase 2b Studies Advancing HBV Functional Cure Strategy Greater China Partnership with Joincare to Accelerate Development of Critical Care Antibiotic soralimixin Strong Cash Position to Pursue New Discovery Opportunities and Partnership Strategy Conference Calls Scheduled: Eng...
Brii Biosciences Announces Licensing Agreement with Joincare Group for Rights to BRII-693 in Greater China
* Joincare Group to lead the clinical development and commercialization of BRII-693 in Greater China * Brii Biosciences retains ex-Greater China rights to address the global antimicrobial resistance threats and continues investment in other priority pipeline assets DURHAM, N.C. and BEIJING, J...
Brii Bio Presents Late-Breaking Data from Its Ongoing Phase 2 ENSURE Study at EASL Congress 2025, Suggesting BRII-179's Role in Advancing Higher HBsAg Loss
* End of treatment (EOT) data from Cohort 4 of the ENSURE study suggest that patients responding to prior BRII-179 treatment achieved faster and higher rate of surface antigen clearance with curative treatments compared to BRII-179naïve participants, strengthening the case for a novel enrichmen...
Brii Bio Unveils New Data from Its Ongoing Phase 2 ENSURE Study at APASL 2025, Showcasing BRII-179's Unique Potential to Prime and Boost Higher HBsAg Loss Through Target Patient Identification
* Preliminary data from Cohort 4 of the ENSURE study supports a novel enrichment strategy to utilize BRII-179 to identify patients who are immune responders and have the potential to achieve higher HBsAg loss at EOT * 48-week EOT data from Cohort 1-3 of the ENSURE study clearly suggests the a...
Brii Biosciences Provides Corporate Update and Reports Full-Year 2024 Financial Results
Strategic Acquisition of Intellectual Property of BRII-179, a Wholly Owned Phase 2b Asset Capable of Combining with Multiple HBV Treatment Modalities for Cure Data from Multiple Ongoing Late-Stage Studies Reinforce Brii Bio's HBV Functional Cure Strategy of Optimized Combination Regimens to Target...
Brii Bio Announces Signing of Asset Purchase Agreements Acquiring BRII-179 IP Rights and Completed Patient Enrollment for ENRICH Study
Further secures Brii's rights in BRII-179 with full control of intellectual property, future manufacturing and supply Several combination treatment studies containing BRII-179 have quickly started to carry forward the Company's strategy for HBV functional cure DURHAM, N.C. and BEIJING, Dec. 31, ...
Brii Bio Delivers First Head-to-Head Comparison of siRNA (Elebsiran) and PEG-IFNα Combination versus PEG-IFNα Alone at AASLD's The Liver Meeting® 2024, Demonstrating Higher Loss of HBV Surface Antigen by Combining with siRNA
48-week end-of-treatment data from the ongoing Phase 2 ENSURE study clearly demonstrated the added benefits of elebsiran towards achieving a higher functional cure rate in combination with PEG-IFNα Data from multiple combination studies sponsored by the Company and its partner continue to suppor...
Brii Bio Presents New Data from Its Ongoing Phase 2 Chronic Hepatitis B Trials at EASL™ Congress 2024
New translational data demonstrate that BRII-179-induced immune responses are associated with high HBsAg reduction in a subset of participants with chronic HBV infection BRII-179 as add-on therapy induces functional antibody responses and contributes toimproved sustained HBsAg loss in PEG-IFNa-t...
Brii Biosciences Announces Two Breakthrough Therapy Designations for BRII-877 and BRII-835 Building on Extensive Clinical Evidence from Multiple Phase 2 Studies
DURHAM, N.C. and BEIJING, May 14, 2024 /PRNewswire/ -- Brii Biosciences Limited
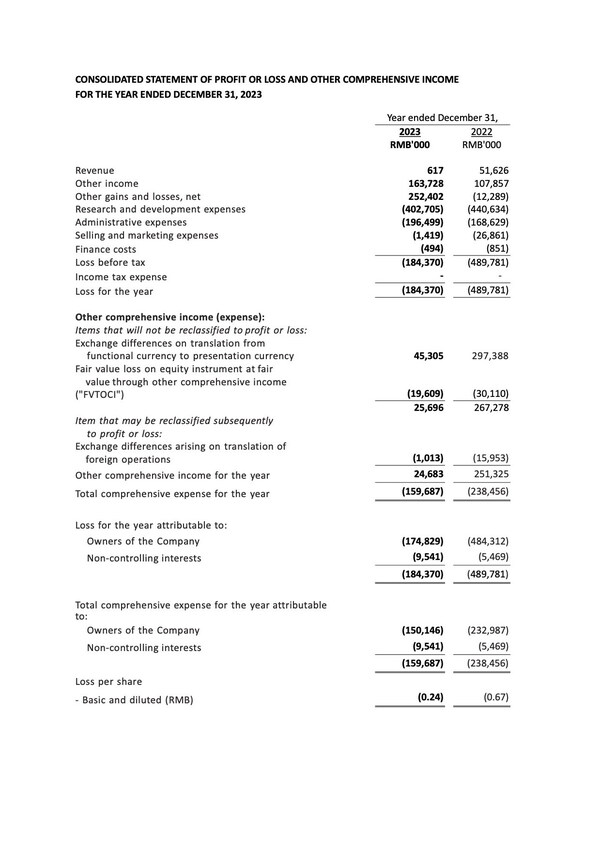
Brii Biosciences Provides Corporate Update and Reports Full-Year 2023 Financial Results
Transitioning HBV cure programs into multiple late-stage combination studies with interim results throughout 2024 and 2025 informing Company's registrational strategy Integrating R&D, manufacturing and commercial upsides by acquiring full intellectual property rights of BRII-179 and expanding it...
Brii Biosciences Announces Agreement to Acquire VBI's IP Rights in BRII-179 (VBI-2601) and Plans to Initiate Technology Transfer to Expand Clinical and Commercial Supplies
Acquiring all intellectual properties relating to BRII-179 and eliminating future milestone and royalty payments to VBI, subject to achievement of certain activities Transferring manufacturing technologies of BRII-179 and PreHevbrio/PreHevbri to additional manufacturing sites and expanding footp...
Brii Biosciences Appoints Dr. Brian Alvin Johns as Chief Scientific Officer
DURHAM, N.C., and BEIJING, Jan. 3, 2024 /PRNewswire/ -- Brii Biosciences Limited
Brii Bio Presents New Data Highlighting Progress Towards Achieving HBV Functional Cure at AASLD's The Liver Meeting® 2023
Direct evidence that BRII-179-induced functional antibody responses can contribute to increased and sustained HBsAg loss rate New insight in utilizing BRII-179 to enriching patients with intrinsic humoral immune responses for higher HBsAg loss or HBV functional cure rates New data support ...
Brii Bio Presents New Data Highlighting Progress Towards Achieving HBV Functional Cure at AASLD's The Liver Meeting® 2023
Direct evidence that BRII-179-induced functional antibody responses can contribute to increased and sustained HBsAg loss rate New insight in utilizing BRII-179 to enriching patients with intrinsic humoral immune responses for higher HBsAg loss or HBV functional cure rates New data support ...
Brii Biosciences Announces Topline Interim Results of Phase 2 Study Evaluating BRII-179 (VBI-2601) in Combination with PEG-IFNα for the Treatment of Chronic Hepatitis B
BRII-179 (VBI-2601) as an add-on therapy to standard of care PEG-IFNα increases HBsAg loss rate at the end of treatment and 12 weeks follow up Significantly increased HBsAg seroconversion rate is strongly associated with BRII-179 treatment and correlates with the increased rate of HBsAg loss Saf...
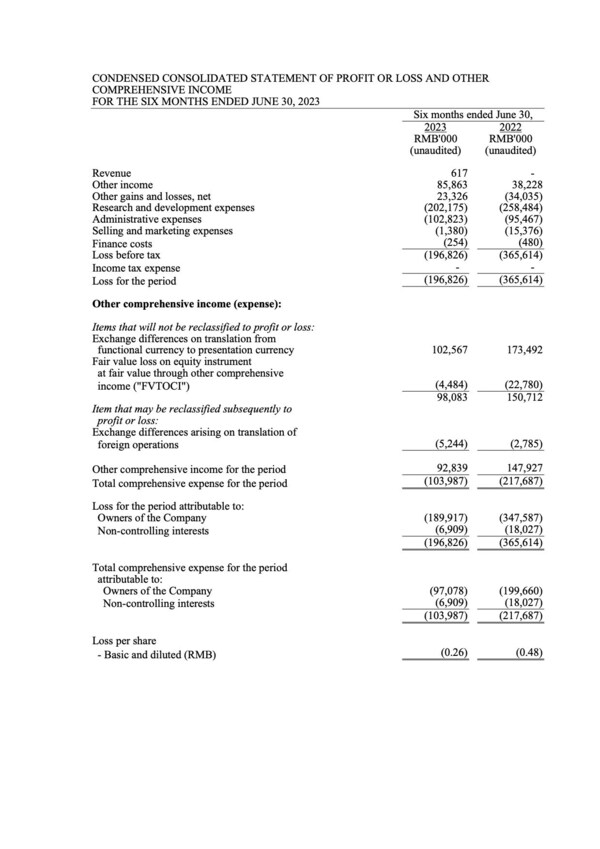
Brii Biosciences Provides Corporate Updates and Reports 2023 Interim Results
First patient dosed in a PEG-IFN-α controlled BRII-835 + PEG-IFN-α combination Phase 2 study for HBV functional cure First of several studies to investigate the potential of BRII-179 in enriching patients with strong intrinsic anti-HBsAg responses for curative treatments to start before the end ...
Brii Biosciences Broadens its Leadership in Hepatitis B with a Portfolio Now Addressing Disease's Burdens from Prevention to Cure
Extension of BRII-179 license from VBI Vaccines to global rights bolsters Brii Bio's position to achieve best-in-class HBV functional cure in broad patient populations Company also acquires exclusive rights to PreHevbri®, a clinically differentiated, 3-antigen prophylactic vaccine, from VBI inGr...
Brii Biosciences Provides Latest Clinical Development and Corporate Updates
Company extends BRII-179 license to global rights and introduces preventive vaccine, PreHevbri® in Greater China and Asia Pacific markets New data from BRII-835 + PEG-IFN-α study have demonstrated that robust anti-HBs antibody responses at the end of treatment are associated with sustained HBsA...
Brii Bio Announces New Data from Partners Underscoring Potential for HBV Functional Cure at EASL™ Congress 2023
Late-breaking data from BRII-835 + PEG-IFN-a study demonstrate anti-HBs titers at the end of treatment were associated with sustained HBsAg loss 24 weeks after the end of treatment New data show more robust and persistent anti-HBs titers following vaccination with VBI's 3-antigen prevention vac...