Brii Biosciences Appoints Taiyin Yang to Board of Directors, Strengthening Product Quality Oversight to Support Company Momentum
Addition increases diversity of Board governance and supports further
independent expertise
DURHAM, N.C. and BEIJING, Sept. 1, 2022 /PRNewswire/ -- Brii Biosciences
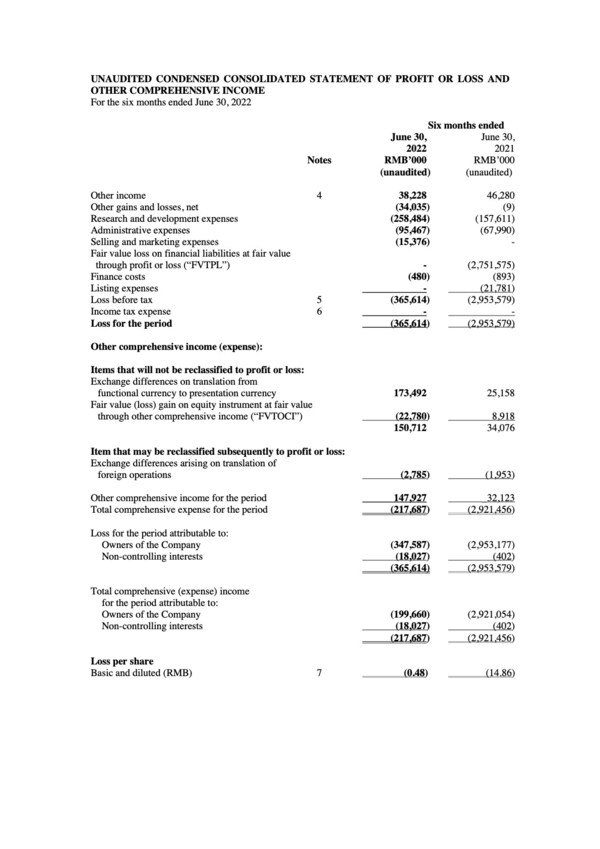
Brii Biosciences Provides Corporate Update and Reports 2022 Interim Results
Additions to executive team strengthen global leadership and position Company for strategic long-term growth First-ever product launch of long-acting amubarvimab/romlusevimab combination therapy for COVID-19 inChina advances Brii Bio from clinical development to commercial stage biotechnology co...
Brii Biosciences Appoints Eleanor de Groot as Chief Technology Officer and Aleksandar Skuban as Central Nervous System Diseases Therapy Area Head
Building a leadership team with global and local experience and expertise to
enable rapid execution across Company's broad therapeutic strategy
DURHAM, N.C. and BEIJING, Aug. 23, 2022 /PRNewswire/ -- Brii Biosciences
Brii Biosciences Announces Positive Data Demonstrating its Long-Acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, Retains Neutralizing Activity Against Live Omicron Virus BA.4/5 and BA.2.12.1 Subvariants
Independent lab results demonstrate that the combination therapy retains neutralizing activity against currently dominant COVID-19 strains, as previously demonstrated with all variants of concern to date The amubarvimab/romlusevimab combination is commercially available in China and is under rev...
Brii Bio Announces Strategic Partnership with China Resources Pharmaceutical Commercial Group to Advance the Commercialization of Long-acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, in China
The partnership will accelerate stockpiling, channel distribution and hospital access for the amubarvimab/romlusevimab combination in China The amubarvimab/romlusevimab combination is the first locally-discovered and approved SARS-CoV-2 target-specific treatment inChina, evaluated through a rand...
Brii Biosciences Appoints Dr. Susannah Cantrell as Chief Business Officer
Strategic executive hire adds to Company's global leadership team and positions
the Company for continued growth
DURHAM, N.C.and BEIJING, July 13, 2022 /PRNewswire/ -- Brii Biosciences
Limited
Brii Biosciences Announces Commercial Launch of its Amubarvimab/Romlusevimab Combination Therapy for COVID-19 in China
The Company is working closely with its commercial partners to supply the combination therapy to patients in need Brii Bio progressed the combination therapy from discovery to global Phase 3 data readout and first regulatory approval by the China NMPA in less than 20 months, validating the Com...
Brii Biosciences Exercises Option for Vir Biotechnology's VIR-3434, a Broadly Neutralizing Monoclonal Antibody Targeting Hepatitis B in Greater China
Exclusive development and commercialization rights to VIR-3434 in China strengthen Company's leadership and robust clinical pipeline in HBV Key partnership with Vir enables multitude of combination treatment options as part of Company's strategic approach to developing a functional cure for HBV ...
Brii Biosciences Announces Positive Data from a Randomized, Single-Blind Study of its Long-Acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, in China
Data are consistent with the results of the global Phase 3 ACTIV-2 trial, demonstrating a favorable safety and tolerability profile in people with both severe and non-severe SARS-CoV-2 infections inChina Clinical benefits were observed in efficacy indicators such as RNA conversion rate, resoluti...
Brii Biosciences to be Added to MSCI China Small Cap Index
DURHAM, N.C. and BEIJING, May 25, 2022 /PRNewswire/ -- Brii Biosciences Limited
Brii Bio Announces Positive Data Demonstrating its Long-Acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, Retains Neutralizing Activity Against Omicron BA.2 Subvariant
* Studies conducted by independent labs demonstrate the amubarvimab/romlusevimab combination retains neutralizing activity against COVID-19 Omicron variant * Review of the monoclonal antibody combination therapy is underway by the U.S. Food and Drug Administration (FDA) for Emergency Use Auth...
Brii Bio Reports Progress on ESG Commitments in Its Inaugural ESG Report
DURHAM, N.C. and BEIJING, May 7, 2022 /PRNewswire/ -- Brii Biosciences Limited
Brii Bio Presents New Data on BRII-835 (VIR-2218) in Chinese Patients with Chronic Hepatitis B at APASL 2022
New data demonstrate BRII-835 (VIR-2218) was well tolerated with notable
reductions in serum HBsAgobserved in the Chinese patients infected with chronic
hepatitis B
DURHAM, N.C. and BEIJING, March 31, 2022 /PRNewswire/ -- Brii Biosciences
Brii Bio Announces Strategic Partnership with Sinopharm to Advance Commercialization of Long-Acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, in China
DURHAM, N.C. and BEIJING, March 30, 2022 /PRNewswire/ -- Brii Biosciences
Limited
Brii Biosciences Provides Corporate Update and Reports Full Year 2021 Financial Results
– Advancing key programs in HBV, CNS, and HIV – – Received first BLA approval for COVID-19 Treatment in China and added to China's latest COVID-19 Diagnosis and Treatment Guidelines– – Multiple clinical value drivers expected across the portfolio in 2022 and beyond – – Cash, bank balances, an...
Brii Bio Announces that National Health Commission of China Adds Amubarvimab/Romlusevimab Combination to its COVID-19 Diagnosis and Treatment Guidelines (9th Pilot Edition)
The amubarvimab/romlusevimab combination is the first locally-discovered and approved SARS-CoV-2 target-specific treatment inChina, through a randomized, double-blind and placebo-controlled trial In vitro tests shows that amubarvimab/romlusevimab combination retains neutralizing activity against...
Brii Bio Appoints Karen D. Neuendorff as Chief People Officer and Head of Human Resources
DURHAM, N.C. and BEIJING, Jan. 27, 2022 /PRNewswire/ -- Brii Biosciences
Brii Bio Doses First Patient in Phase 2a/2b Clinical Trial of BRII-179 (VBI-2601) for the Functional Cure of Chronic Hepatitis B
DURHAM, N.C. and BEIJING, Dec. 27, 2021 /PRNewswire/ -- Brii Biosciences
Brii Bio Announces Amubarvimab/Romlusevimab Combination Retains Neutralizing Activity Against Omicron SARS-CoV-2 Variant
DURHAM, N.C. and BEIJING, Dec. 12, 2021 /PRNewswire/ -- Brii Biosciences
Brii Bio Announces Amubarvimab/Romlusevimab Combination Received Approval from NMPA as First COVID-19 Neutralizing Antibody Combination Therapy in China
The approval marks the first locally-discovered and approved SARS-CoV-2 target-specific treatment inChina, through a randomized, double-blind and placebo-controlled trial Brii Bio is also seeking U.S. FDA Emergency Use Authorization for the amubarvimab/romlusevimab combination DURHAM, N.C. and ...