Nona Biosciences Appoints Steve Arkinstall, D. Phil., as Chief Scientific Advisor
CAMBRIDGE, Mass., June 6, 2023 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited, committed to cutting-edge antibody technology innovation and provider of integrated solutions from "Idea to IND" (I to I™), today announced the appointment of Steve Arkinstall, D.Ph...
Harbour BioMed Announces First Patient Dosed in Phase I Study of First-in-Class Anti-B7H7 (HHLA2) Antibody HBM1020
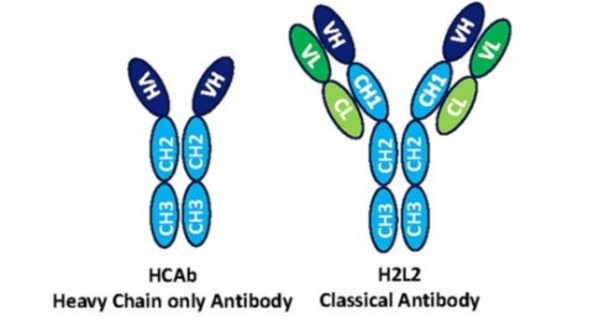
* HBM1020 is a first-in-class therapeutic monoclonal antibody against B7H7/HHLA2 entering clinical development globally. * HBM1020 was generated from Harbour Mice® H2L2 transgenic mice platform. * HBM1020 has great potential to address huge unmet medical needs in patients with advanced malig...
Harbour BioMed Reports Results of Phase Ib Clinical Trial of Porustobart in Combination of Toripalimab in Patients with Hepatocellular Carcinoma at ASCO 2023
* Porustobart in combination of toripalimab showed promising anti-tumor activity * Porustobart in combination of toripalimab showed acceptable safety profile in HCC * Porustobart in combination of toripalimab showed favorable PK/PD signature CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZH...
Nona Biosciences Announces Strategic Collaboration Agreement with PharmaEssentia Innovation Research Center
CAMBRIDGE, Mass., May 11, 2023 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited, committed to cutting-edge technology innovation and provider of integrated solutions from "Idea to IND" (I to I™), announced today it has entered into a strategic collaboration agre...
Nona Biosciences and Washington University Join Forces to Tackle Emerging RNA Viruses
CAMBRIDGE, Mass., April 18, 2023 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited, committed to cutting edge technology innovation and provider of integrated solutions from "Idea to IND" (I to ITM), announced today it has entered into a collaboration agreement w...
Nona Biosciences Enters into Collaboration Agreement with ExeVir Bio
CAMBRIDGE, Mass., April 5, 2023 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited, committed to cutting-edge technology innovation and provider of integrated solutions from "Idea to IND" (I to I™ ), announced today it has entered into a collaboration agreement wi...
Harbour BioMed Reports Full Year 2022 Financial Results
CAMBRIDGE, Mass., SUZHOU, China and ROTTERDAM, Netherlands, April 3, 2023 /PRNewswire/ -- Harbour BioMed ("HBM" or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development and commercialization of novel antibody therapeutics focusing on immune-oncolo...
Harbour BioMed Announces Positive Topline Results from Phase III Trial of Batoclimab for Treatment of Generalized Myasthenia Gravis
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, March 6, 2023 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
HBM Alpha Therapeutics Raises Seed Round to Advance Next-Gen Therapies for Endocrine Disorders
CAMBRIDGE, Mass., Jan. 26, 2023 /PRNewswire/ -- HBM Alpha Therapeutics (HBMAT), Inc., an innovative biotechnology company incubated byHarbour BioMed (HKEX: 02142), announced that it completed seed financing to advance its leading programs, novel antibody therapies to treat congenital adrenal hype...
Nona Biosciences Enters into HCAb Based Drug Discovery Collaboration Agreement with Dragonfly Therapeutics
CAMBRIDGE, Mass., Nov. 21, 2022 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited committed to providing a total solution from "Idea to IND" ("I to ITM"), today announced that it has entered into a collaboration agreement with Dragonfly Therapeutics based on Nona...
Nona Biosciences Announces Attendance at PEGS Europe 2022
CAMBRIDGE, Mass., Nov. 15, 2022 /PRNewswire/ -- Nona Biosciences (a wholly-owned subsidiary of HBM Holdings, HKEX: 02142) announced that the Company is attending the 14th Protein and Antibody Engineering Summit (PEGS) in Barcelona, Spain, November 14-16, 2022. The booth is located at #54. Frank ...
HARBOUR BIOMED LAUNCHES NONA BIOSCIENCES' "IDEAS TO IND" PRECLINICAL SOLUTIONS BUSINESS TO ACCELERATE GLOBAL BIOTHERAPEUTIC INNOVATION
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Nov. 14, 2022 /PRNewswire/ -- Harbor BioMed (HKEX: 02142) today announced establishment of a wholly-owned subsidiary, Nona Biosciences, to provide next-generation antibody and biotherapeutic solutions to partners from discovery to IND. T...
Harbour BioMed Enters into a License and Collaboration Agreement with Moderna
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Nov. 10, 2022 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142) today announced that Nona Biosciences, a subsidiary wholly owned by the Company, had entered into a license and collaboration agreement with ModernaTX, Inc. (NASDA...
Harbour BioMed Announces Dosing of First Patient in Phase I Trial of B7H4x4-1BB Bispecific Antibody in the United States
CAMBRIDGE, Mass., ROTTERDAM and SUZHOU, China, Oct. 20, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) announced that, it has successfully completed the dosing of first patient in the global phase I trial of B7H4x4-1BB bispecific antibody HBM7008 inthe United States. This study will eva...
Harbour BioMed Announces Upcoming Poster Presentations at the 37th Society for Immunotherapy of Cancer Annual Meeting
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Oct. 13, 2022 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immunol...
Harbour BioMed Announces First Subject Dosed in Phase I Study of Next-Gen Anti-TSLP Fully Human Monoclonal Antibody
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Sept. 27, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) today announces that it has completed the first subject dosing in a phase I study of HBM9378 (or SKB378 as referred to by Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd...
Harbour BioMed to Announce 2022 Interim Results on August 31, 2022
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 9, 2022 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142) announced that it will report financial results for the first half year endedJune 30, 2022, on Wednesday, August 31, 2022. The Company will host an English virtual ...
PNAS Published Preclinical Results of Harbour BioMed's Next-Generation Fully Human Heavy-chain Antibody Porustobart
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 8, 2022 /PRNewswire/ -- Harbour BioMed (the "Company", HKEX: 02142) announced that the preclinical results of porustobart (HBM4003,or the HCAb 4003-2 in the research paper), a next-generation fully human heavy-chain antibody with a ...
Harbour BioMed Announces IND Clearance for B7H4x4-1BB Bispecific Antibody by the U.S. Food and Drug Administration
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, June 27, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) announced that the U.S. Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for the B7H4x4-1BB bispecific antibody (HBM7008), whi...
Harbour BioMed Announces IND Approval for B7H4x4-1BB Bispecific Antibody
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, June 8, 2022 /PRNewswire/ --Harbour BioMed ("HBM", HKEX: 02142) announced that China National Medical Products Administration (NMPA) had approved the investigational new drug (IND) application to commence phase I trial of its B7H4x4-...