Daewoong Pharmaceutical submitted NDA for its global new drug Fexuclue in 11 countries, only 1 year after approval in Korea
"Expected to change GERD drug market landscape" * Application for product approval of Fexuclue, a new drug for gastroesophageal reflux disease (GERD), has been completely submitted toColombia ,Vietnam, and Saudi Arabia * NDA submissions in 11 countries completed in the shortest time among K...
Daewoong Pharmaceutical announces success in developing a new antidiabetic medication and its aims to enter the market in over 50 countries by 2030
* Delivers new drug for two consecutive years by obtaining product license on "Envlo", Korea's 36th new drug from the Ministry of Food and Drug Safety * Plans to enter the type 2 diabetes global market worth $71.4 billion * Provides new and broad treatment options for patients with insufficie...
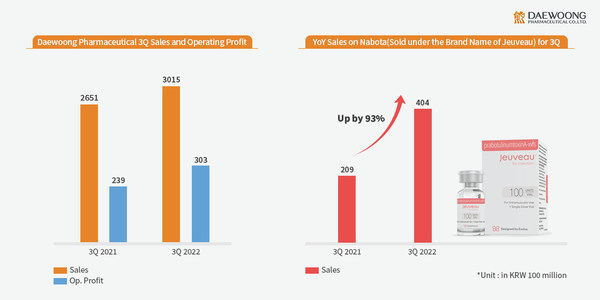
Daewoong Pharmaceutical Surpasses KRW 300 Billion in Q3 Revenue as Nabota Sales Grew by 93%, Leading Overall Growth in Revenue
* Nabota exports grew significantly, achieving a 93.3% growth YOY, and overall revenue topsKRW 40.4 billion * Fexuclue, a new drug released last July, shows steep growth in market share in Korea * Nabota enters the European market and Fexuclue enters wide use as in prescriptions for gastrit...
Daewoong Pharmaceutical Presents Phase 3 Clinical Trial Results of New Diabetes Treatment 'Enavogliflozin' and Roadmap to Enter 50 Countries by 2030
* Highly anticipated to be an effective therapeutic for its excellent efficacies in reducing blood glucose levels and safety SEOUL, South Korea, Nov. 2, 2022 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong), the South Korea -based global healthcare company, announced the phase III clinical tri...
CGBio Signing a North America Out-license Contract on "Novosis rhBMP-2," a Bone Substitute with Orthofix, USA
* Embarking on a successful journey of exporting its home-grown product technology toNorth America, the largest market for bone substitutes * Enabling fast bone formation by applying bone morphogenetic protein (rhBMP-2) and next-generation synthetic carrier SEOUL, South Korea and LEWISVILLE, ...
Daewoong Pharmaceutical Co., Ltd. revs up the growth engine for Nabota, achieving the highest quarterly performance ever
- Daewoong Pharmaceutical Co., Ltd. achieved 293.8 billion won in sales and 33.6 billion won in operating revenue in the second quarter due to the growth of ethical drugs and Nabota. - A continuous increase in profitability is expected due to sales visualization of the new drug Fexuclue and Na...
Daewoong Pharmaceutical Gets First Korean US FDA Fast-Track for New Idiopathic Pulmonary Fibrosis Drug
* US Food and Drug Administration (FDA) fast-tracks idiopathic pulmonary fibrosis treatment DWN12088 * First-in-class new drug to quickly take on the pulmonary fibrosis market, predicted to reach$6.1 billion globally by 2030 SEOUL, South Korea, July 20, 2022 /PRNewswire/ -- As many Korean p...
Daewoong Pharmaceutical begins multinational phase 2 clinical trial for DWN12088, a new drug for idiopathic pulmonary fibrosis
- U.S. Food and Drug Administration (FDA) approved the IND for the phase 2 clinical trial for patients with idiopathic pulmonary fibrosis - Daewoong Pharmaceutical to start a multinational phase 2 clinical trial for DWN12088 in September SEOUL, South Korea, June 24, 2022 /PRNewswire/ -- Daewoong...
Daewoong Pharmaceutical announced first-quarter 2022 results
- Marked sales of KRW 272.2 billion and operating profit of KRW 26.8 billion - Reported record-high quarterly operating profit, thanks to highly profitable ETC drugs and export growth of Nabota - Expected to see increasing profitability and robust growth due to export expansion of Nabota and the...
HanAll Biopharma and Daewoong Pharmaceutical Invest in Turn Biotechnologies to Expand Growth Initiative
* HanAll Biopharma and Daewoong Pharmaceutical invest in the broad potential of Turn Biotechnologies * Turn has developed a novel cell rejuvenating platform with significant potential for application in an array of age-related diseases SEOUL, South Korea ,April 12, 2022 /PRNewswire/ -- HanAll...
/DISREGARD RELEASE: HanAll Biopharma and Daewoong Pharmaceutical/
We are advised by HanAll Biopharma and Daewoong Pharmaceutical that journalists and other readers should disregard the news release, "Daewoong Pharmaceutical and HanAll Biopharma Invest in Turn Biotechnologies to Expand Growth Initiative", issued11-Apr-2022 over PR Newswire. ...
Daewoong Pharmaceutical submitted NDA for its global new drug Fexuclue Tablets to the Philippines
- From Last February to mid-March, NDA has been submitted to each country sequentially - Daewoong Pharmaceutical expects to strengthen its global business in key markets of ASEAN countries by obtaining marketing authorization in foreign countries with its branch offices SEOUL, South Korea, Marc...
Daewoong Pharmaceutical Announces Successful Phase 3 Topline Results for New Antidiabetic Drug's Triple Combination Therapy
SEOUL, South Korea, Feb. 25, 2022 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) announced the topline results of the phase 3 clinical trial for a triple combination therapy of Enavogliflozin, a new antidiabetic drug with the mechanism of SGLT-2 inhibitor, with Metformin and Gemigliptin. Enav...
Daewoong Pharmaceutical Posts Record Annual Revenue in 2021, with Export Growth of Nabota and New Drug Development
* Recorded operating profit KRW 88.9 billion and net profit KRW 31.6 billion in 2021 with the outcome of its relentless focus on R&D * Attributed to export upswing of Nabota, KRW 1.1 trillion overseas technology transfer of Fexuclue tablets, and growth of highly profitable ETC drugs SEOUL, So...
Daewoong Pharmaceutical Released Positive Phase 3 Topline Results for New Antidiabetic Drug
SEOUL, South Korea, Jan. 25, 2022 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) has confirmed promising phase 3 topline results that focuses on the therapeutic effects as an Enavogliflozin monotherapy and a combination therapy with Metformin.Daewoong's Enavogliflozin is an SGLT-2 inhibitor i...
Maypharm Launches a New Professional set HAIRNA for Hair Loss Solution
SEOUL, South Korea and NEW YORK, Dec. 24, 2021 /PRNewswire/ -- Maypharm is known as a manufacturer of recently successfully launched Metox botulinum toxin with a 2nd generation technological advantage and meNnus PLA filler for collagen regeneration, now is focusing on a new professional set HAIRN...
Maypharm Launches New PLA meNnus for Collagen Regeneration
SEOUL, South Korea and NEW YORK, Nov. 5, 2021 /PRNewswire/ -- Maypharm Co., Ltd is excited to announce the launch of a new PLAmeNnus (200mg/vial), which is available at Maypharm website & wholesale. MENNUS is a new combination of mesotherapy and biotechnology directed to fill shelves of profes...
Daewoong's "Leaps and Bounds" Q3 Performance, Exceeds KRW 20B in Operating Income for Three Consecutive Quarters
* Advancement of Nabota and prescriptions... KRW 290.6B sales and KRW 22.7B operating income * Expanding growth momentum with the release of Fexuprazan in Korea and Nabota inEurope next year SEOUL, South Korea, Nov. 2, 2021 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) (CEOSengho Jeon) a...
CGBIO to introduce AI-empowered robotic intervention device, with clinical trials
* CGBIO, NDR Medical Technology, forge a partnership in a move to introduce puncture robot technology and collaborate in clinical studies. * CGBIO expects this partnership to gain its stronger competitiveness in the global digital healthcare market beyond the presence as a pioneer of AI based ...
Daewoong Pharmaceutical and Hanall Biopharma Invest $1M USD in Alloplex Biotherapeutics
SEOUL, South Korea, Aug. 19, 2021 /PRNewswire/ -- Daewoong Pharmaceutical and
Hanall Biopharma ofSouth Korea are expanding their global open collaboration
initiative by investing in Alloplex Biotherapeutics, an emergingBoston-based
biotechnology company.