Daewoong Pharmaceutical Announces Camostat Achieving 50% Faster Recovery Time for Mild COVID-19 Patients Over Age of 50 in Topline Results from Phase 2B Clinical Trial
- Improvement of symptoms such as cough and dyspnea in mild patients over age 50 was reported to be as twice as fast and statistically significant in the treatment group (4 days) compared to the placebo control group (9 days). - Development is underway for potential combination therapies and nove...
Daewoong Pharmaceutical Publishes Results of First Clinical Study of SGLT-2 Inhibitor 'Enavogliflozin' for Canine Diabetes
SEOUL, South Korea, June 1, 2021 /PRNewswire/ -- Daewoong Pharmaceutical (CEO Sengho Jeon) announced that the results of an investigator-initiated clinical study on the effect of Enavogliflozin, originally developed as Type II Diabetes Treatment forhumans, on treating canine diabetes were publish...
Daewoong, first-in-class PRS-inhibitor for Idiopathic Pulmonary Fibrosis(IPF) shows promising result in early stage clinical test
* We confirmed the safety of 'DWN12088' and secured grounds for establishing treatment dosage * Confirming the potential as a treatment for IPF and planning to apply for Ph.2 within 2021 * 2nd ODD granted as treatment for systemic sclerosis from the US FDA SEOUL, South Korea, May 27, 2021...
Daewoong, first-in-class PRS-inhibitor for Idiopathic Pulmonary Fibrosis (IPF) shows promising result in early stage clinical test
* We confirmed the safety of 'DWN12088' and secured grounds for establishing treatment dosage * Confirming the potential as a treatment for IPF and planning to apply for Ph.2 within 2021 * 2nd ODD granted as treatment for systemic sclerosis from the US FDA SEOUL, South Korea, May 24, 2021 /...
Daewoong showed improved performance both sales and earnings in Q1
* OP. income exceeded 20 billion won due to successful export of Fexuprazan and sales recovery * Nabota(botulinum toxin) revenue soared after settlement, historical high performance in March * Turnaround in earnings just started and will continue to 2H SEOUL, South Korea, May 7, 2021 /PRNew...
Daewoong Pharmaceutical Announces Publication of Novel P-CAB Fexuprazan Phase 1 Bridging Study
SEOUL, South Korea, Nov. 6, 2020 /PRNewswire/ -- On October 27, the bridging study results on Fexuprazan, developed by the Korean pharmaceutical company, Daewoong Pharmaceutical (Daewoong; CEOSengho Jeon), was published in the SCI medical journal Alimentary Pharmacology and Therapeutics (AP&T). F...
Daewoong Pharmaceutical Partners with Tufts Medical Center for Phase 2 Clinical Trial with Niclosamide
SEOUL, South Korea, Oct. 29, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (CEO Sengho Jeon, KRX: 069620) announced on October 26 that the collaborative clinical research agreement between Daewoong and Tufts Medical Center was signed to prepare for the phase 2 clinical study of DWRX2003 (active i...
Daewoong Pharmaceutical's COVID-19 Treatment Candidate Also Highly Effective against Influenza Virus
SEOUL, South Korea, Oct. 28, 2020 /PRNewswire/ -- Daewoong Pharmaceutical, a South Korean pharmaceutical giant announced that its Covid-19 treatment candidate of sustained-release niclosamide (DWRX2003) also showed promising study results for a fight against the upcoming 'Twindemic' involving COV...
Daewoong Pharmaceutical's Long-Acting Niclosamide Shows High Promise Against Upcoming COVID-19 'Twindemic'
SEOUL, South Korea, Oct. 27, 2020 /PRNewswire/ -- Daewoong Pharmaceutical, a South Korean pharmaceutical giant announced that its Covid-19 treatment candidate of sustained-release niclosamide (DWRX2003) also showed promising study results for a fight against the upcoming 'Twindemic' involving COV...
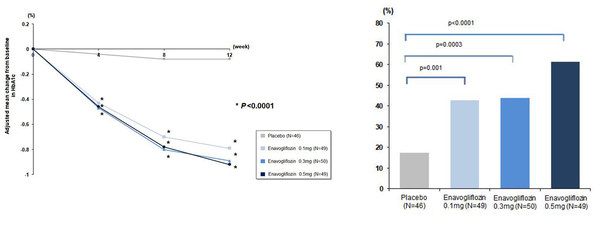
Daewoong Pharmaceutical's new SGLT2 inhibitor for diabetes treatment demonstrates remarkable effect in phase 2 clinical trial
SEOUL, South Korea, Sept. 29, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) (CEOSengho Jeon) announced the result of its phase 2 trial on enavogliflozin, a novel SGLT2 inhibitor, for the first time at the 2020 International Congress of Diabetes and Metabolism (ICDM) held on Sep. 18–19. ...
Daewoong Pharmaceutical announces Korea's first-ever SGLT2 inhibitor for diabetes treatment
* Daewoong Pharmaceutical's enavogliflozin to demonstrate remarkable blood glucose lowering effect and favorable safety in type 2 diabetic patients through phase 2 clinical trial * Presented and demonstrated the result of phase 2 clinical trial in Korean patients with type 2 diabetes at the i...
Daewoong Pharmaceutical Acquires Halal Certification for 'Easyef' through Daewoong Infion
SEOUL, South Korea and JAKARTA, Indonesia, May 27, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (CEOSengho Jeon) announced on May 25 that the Indonesian joint venture Daewoong Infion (CEOChang Woo Suh) obtained Halal certification for the diabetic foot ulcer drug 'Easyef topical solution(Easyef)...
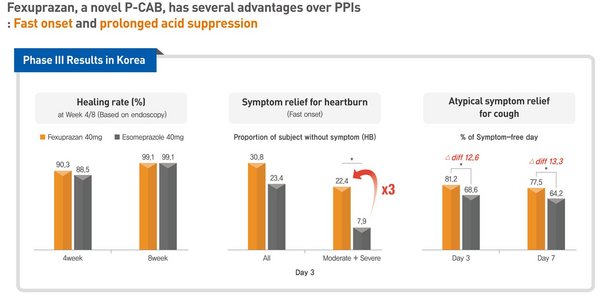
Daewoong Pharmaceutical Unveils Phase 3 Clinical Data of Fexuprazan, A Novel Potassium-competitive Acid Blocker
SEOUL, South Korea, May 11, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (Daewoong) unveiled for the first time the phase 3 clinical data of Fexuprazan, a novel gastroesophageal reflux disease agent at Digestive Disease Week (DDW) 2020. The abstract of Fexuprazan has been rated in the top 10% pos...