I-Mab Announces Poster Presentations of 4-1BB Bispecific Antibody Portfolio at SITC 2023
ROCKVILLE, MD, U.S. and SHANGHAI, China, Nov. 1, 2023 /PRNewswire/ -- I-Mab (Nasdaq: IMAB) (the "Company"), a global biotechnology company focused on bringing highly differentiated medicines to patients around the world through the discovery, development, and commercialization of novel immunother...
I-Mab Announces Phase 1 Data of Givastomig at ESMO 2023
ROCKVILLE, Md. and SHANGHAI, China, Oct. 16, 2023 /PRNewswire/ -- I-Mab (Nasdaq: IMAB) (the "Company"), a global biotechnology company focused on bringing highly differentiated medicines to patients around the world through the discovery, development, and commercialization of novel immunotherapie...
I-Mab and ABL Bio Announce Latest Updates of PD-L1 and 4-1BB Bispecific Antibody TJ-L14B/ABL503
- Among 14 efficacy-evaluable patients in the ongoing Phase 1 study, one achieved a complete response, one had partial response and two unconfirmed partial responses; maximum tolerated dose not yet reached - Patent rights secured for TJ-L14B/ABL503 in Eurasia until 2039 ROCKVILLE, ...
I-Mab Announces Upcoming Participation at September Conferences
ROCKVILLE, Md. and SHANGHAI, Sept. 5, 2023 /PRNewswire/ -- I-Mab (Nasdaq: IMAB) (the "Company"), is a global biotechnology company focused on bringing highly differentiated medicines to patients around the world through the discovery, development, and commercialization of novel immunotherapies an...
I-Mab Provides Mid-Year 2023 Financial Results, Business and Corporate Updates
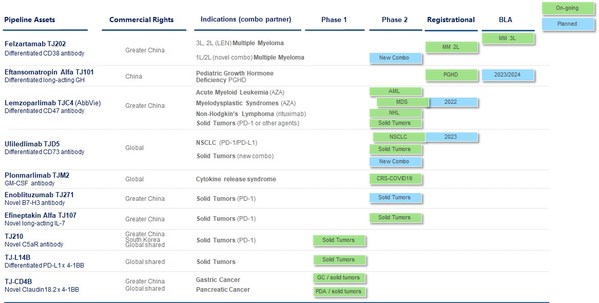
* Significant progress made year-to-date on key clinical assets: ‐ Uliledlimab (CD73 antibody): Encouraging early results were presented at ASCO 2023 ‐ Givastomig (Claudin 18.2 x 4-1BB bispecific antibody): Topline Phase 1 data with promising early efficacy signals, including pat...
I-Mab to Report Mid-Year 2023 Financial Results, Business and Corporate Updates on August 17, 2023
ROCKVILLE, Md. and SHANGHAI, Aug. 8, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of pioneering immunotherapies, today announced that it will report business and corporate updat...
I-Mab Announces Publication of Claudin18.2 x 4-1BB Bispecific Antibody Givastomig in JITC
GAITHERSBURG, Md. and SHANGHAI, July 5, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of pioneering immunotherapies, today announced the publication of a manuscript entitled "C...
I-Mab Announces the Appointment of Raj Kannan as CEO
GAITHERSBURG, Md. and SHANGHAI, June 22, 2023 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of pioneering immunotherapies, today announced the appointment of Mr. Raj Kannan...
I-Mab Announces Encouraging Phase 1b/2 Study Results of Patients with Advanced NSCLC Receiving Uliledlimab and Toripalimab Combination Therapy at ASCO 2023
- Uliledlimab is a differentiated monoclonal antibody designed to target CD73 and promote stronger activation ofpatients' immune system against cancer cells. - In newly diagnosed patients who were not eligible for or declined chemotherapy treatment, 63% of the patients with CD73High and PD-L1 T...
I-MAB Filed 2022 Annual Report on Form 20-F
GAITHERSBURG, Md. and SHANGHAI, May 1, 2023 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it has filed its annual report on Form 20...
I-Mab Announces Poster Presentation of Proprietary CD73 Antibody Uliledlimab at ASCO 2023
GAITHERSBURG, Md. and SHANGHAI, April 26, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that a poster featuring the latest clinical data of ul...
I-Mab Announces Positive Outcome in Arbitration Relating to Agreements with Tracon
* Award is an important victory for I-Mab and its shareholders on multiple levels, ending the multi-year long dispute * Tribunal completely denied Tracon's groundless damages claim of over $200 million relating to I-Mab's next-generation bi-specific antibody assets and awarded no damages to T...
I-Mab Announces First Patient Dosed in Phase 3 Registrational Study of CD47 Antibody Lemzoparlimab in MDS in China
GAITHERSBURG, Md. and SHANGHAI, April 24, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the first patient in a Phase 3 registrational tri...
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Year Ended December 31, 2022
* Strategic reprioritization of the Company's pipeline to focus on key clinical assets and overall streamlining of corporate structure and workforce, resulting in a significant reduction in the cash burn rate and in significant pipeline achievements by focus * Thirteen key clinical milestones...
I-Mab to Report Full Year 2022 Financial Results and Provide Corporate Update on March 31, 2023
GAITHERSBURG, Md. and SHANGHAI, China, March 20, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the Company will report financial results ...
MSCI ESG Updated I-Mab to "A" Rating
GAITHERSBURG, MD. and SHANGHAI, March 7, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that I-Mab has been granted "A" rating by MSCI (Morgan ...
I-Mab Announces Two Poster Presentations of CD47 Antibody Lemzoparlimab at ASH 2022
GAITHERSBURG, MD. and SHANGHAI, Nov. 3, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that two poster presentations featuring preclinical and ...
I-Mab Announces Approval from China CDE to Initiate Phase 3 Registrational Study of Lemzoparlimab in Combination with Azacitidine in Higher-Risk Myelodysplastic Syndrome
GAITHERSBURG, Md. and SHANGHAI, Sept, 13, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it has successfully completed an End-of-Phase 2 (...
I-Mab Announces Positive Phase 2 Data of Lemzoparlimab in Combination with Azacitidine (AZA) in Patients with Higher Risk Myelodysplastic Syndrome at ESMO 2022
* Lemzoparlimab combined with AZA showed encouraging clinical response in higher-risk MDS patients * For patients received initial dose over 3 months, the ORR is 80.6% and for patients received initial dose over 6 months the ORR is 86.7%, CR rate 40% * Lemzoparlimab does not require priming ...
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Six Months Ended June 30, 2022
* Seven key clinical milestones achieved year-to-date, including positive data readouts for lemzoparlimab, uliledlimab, and TJ-CD4B * Lemzoparlimab is on track for Phase 3 study for 1L MDS * Amendment to the global partnership with AbbVie for certain new CD47 antibodies currently in developm...