MSCI ESG Updated I-Mab to "A" Rating
GAITHERSBURG, MD. and SHANGHAI, March 7, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that I-Mab has been granted "A" rating by MSCI (Morgan ...
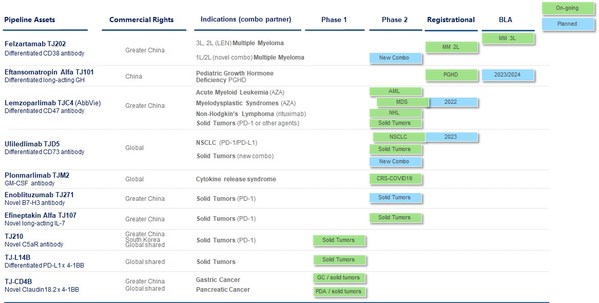
I-Mab Announces Two Poster Presentations of CD47 Antibody Lemzoparlimab at ASH 2022
GAITHERSBURG, MD. and SHANGHAI, Nov. 3, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that two poster presentations featuring preclinical and ...
I-Mab Announces Approval from China CDE to Initiate Phase 3 Registrational Study of Lemzoparlimab in Combination with Azacitidine in Higher-Risk Myelodysplastic Syndrome
GAITHERSBURG, Md. and SHANGHAI, Sept, 13, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it has successfully completed an End-of-Phase 2 (...
I-Mab Announces Positive Phase 2 Data of Lemzoparlimab in Combination with Azacitidine (AZA) in Patients with Higher Risk Myelodysplastic Syndrome at ESMO 2022
* Lemzoparlimab combined with AZA showed encouraging clinical response in higher-risk MDS patients * For patients received initial dose over 3 months, the ORR is 80.6% and for patients received initial dose over 6 months the ORR is 86.7%, CR rate 40% * Lemzoparlimab does not require priming ...
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Six Months Ended June 30, 2022
* Seven key clinical milestones achieved year-to-date, including positive data readouts for lemzoparlimab, uliledlimab, and TJ-CD4B * Lemzoparlimab is on track for Phase 3 study for 1L MDS * Amendment to the global partnership with AbbVie for certain new CD47 antibodies currently in developm...
I-Mab Announces Share Purchase Plans by the Company and the Senior Management
GAITHERSBURG, Md. and SHANGHAI, Aug. 23, 2022 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it plans to implement share repurchases...
I-Mab to Report Business and Corporate Updates and Financial Results for the Six Months Ended June 30, 2022 on August 30, 2022
- Major updates will focus on global clinical development of core assets, upcoming milestones and financial results - The Company will update on potential implementation of a comprehensive share purchase program by the Company and current shareholders - Conference Calls Scheduled at 7:00 a.m....
I-Mab to Present Phase 2 Clinical Data of CD47 Antibody Lemzoparlimab at ESMO Congress 2022
GAITHERSBURG, Md. and SHANGHAI, July 18, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that an abstract reporting the latest clinical data fro...
I-Mab to Host 2022 R&D Day
GAITHERSBURG, MD. and SHANGHAI, July 6, 2022 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it will host its 2022 Research and Devel...
I-Mab Receives Top Rankings in Five Categories by Institutional Investor
GAITHERSBURG, Md. and SHANGHAI, June 23, 2022 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that it was ranked among the top companies i...
I-Mab Partner MorphoSys Announces New License Agreements for Felzartamab and TJ210
SHANGHAI and GAITHERSBURG, Md., June 15, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced two assets the Company has licensed from partner Morpho...
I-Mab Announces Completion of Patient Enrollment in Phase III Clinical Trial of Eftansomatropin alfa for Treatment of Pediatric Growth Hormone Deficiency
SHANGHAI and GAITHERSBURG, Md., May 31, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced the completion of patient enrollment in a Phase 3 clinica...
I-Mab Reports Latest Phase 2 Clinical Data of its Differentiated CD73 Antibody Uliledlimab
- Uliledlimab appears safe and well-tolerated as a monotherapy and a combination therapy with toripalimab with no dose limiting toxicity - Encouraging efficacy signals were observed in a non-small cell lung cancer (NSCLC) patient cohort - Results indicate CD73 expression correlates with clini...
I-Mab to Hold Investor Call to Report Latest Phase 2 Clinical Data of its Differentiated CD73 Antibody Uliledlimab
SHANGHAI and GAITHERSBURG, MD., May 19 , 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq" IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that it will hold a call with investors at 8 a.m. EST on...
I-Mab Provides Updates on Status under Holding Foreign Companies Accountable Act
SHANGHAI and GAITHERSBURG, Md., May 5, 2022 /PRNewswire/ -- I-Mab ("I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today provided updates on its status under the Holding Foreign...
I-Mab Announces Appointments of Richard Yeh as Chief Operating Officer and John Hayslip as Chief Medical Officer
- Biotech and Wall Street veteran Mr. Richard Yeh to focus on strategic investor interactions, global alliance management and facilities as COO - Renowned oncology expert Dr. John Hayslip to further accelerate global clinical development of I-Mab's innovative pipeline as CMO SHANGHAI and GAITHE...
I-Mab Announces Voluntary Lock-ups by Key Shareholders and Senior Management for Long-Term Commitment
SHANGHAI and GAITHERSBURG, Md., March 31, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the Company's key shareholders including CBC Grou...
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Year Ended December 31, 2021
* Financial results demonstrate strong fundamentals * Twenty key clinical milestones achieved year-to-date, including positive data readouts for lemzoparlimab, uliledlimab and felzartamab * Seven business development deals, including a US$315M strategic commercial partnership with Jumpcan on...
I-Mab to Report Full Year 2021 Financial Results and Provide Corporate Update on March 29, 2022
SHANGHAI and GAITHERSBURG, MD, March 18, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the Company will report financial results for the ...
I-Mab Provides Updates Regarding Holding Foreign Companies Accountable Act
SHANGHAI and GAITHERSBURG, Md., March 15, 2022 /PRNewswire/ -- I-Mab ( "I-Mab" or the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today provides updates on the recent development relating ...