Medical/Pharmaceuticals
Yuyu Pharma Establishes UCLA Office to Accelerate U.S. Pet Industry Expansion
SEOUL, South Korea, Jan. 14, 2026 /PRNewswire/ -- Yuyu Bio and Mervyn's Petcare, pet-focused subsidiaries of Yuyu Pharma, have opened a new office on theUCLA campus, strengthening the company's presence in the United States pet and animal health market. The two companies are now based at Magni...
Metabolon Appoints Celia Reyes as Vice President of Human Resources
Celia Reyes will lead Metabolon's global people strategy as the company continues to scale in the evolving multiomics marketplace MORRISVILLE, N.C., Jan. 14, 2026 /PRNewswire/ -- Metabolon, Inc., the global leader in providing metabolomics solutions advancing a wide variety of life science resea...
NYSE Content Advisory: Pre-Market Update + Inaugural U.S.-Saudi Biotech Alliance Summit Begins in SF
NEW YORK, Jan. 14, 2026 /PRNewswire/ -- The New York Stock Exchange (NYSE) provides a daily pre-market update directly from the NYSE Trading Floor. Access today's NYSE Pre-market update for market insights before trading begins. NYSE Content Advisory: Pre-Market Update + Inaugural U.S.-Saudi ...
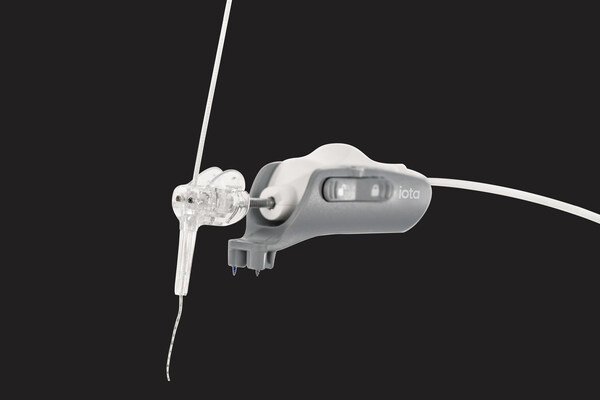
iotaMotion Receives FDA Clearance for Expanded Pediatric Use of iotaSOFT® Robotic-Assisted Cochlear Implant Insertion System
ST. PAUL, Minn., Jan. 14, 2026 /PRNewswire/ -- iotaMotion, Inc
Lynx Analytics Introduces Lumen, an Agentic AI Framework to Improve Decision-Making in Life Sciences
LOS GATOS, Calif., Jan. 14, 2026 /PRNewswire/ -- Lynx Analytics today announced
Lumen
Head-to-Head Real-World Data: Cadonilimab Outperforms PD-1 Inhibitors in First-Line PD-L1-Low Gastric Cancer
HONG KONG, Jan. 13, 2026 /PRNewswire/ -- Akeso, Inc. (9926.HK) announced the presentation of a real-world study at the 2026 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI), comparing cadonilimab plus chemotherapy against a PD-1 inhibitor plus chemotherapy for th...
Enhanced Genetype colorectal cancer risk test released - supports earlier disease detection and ColoSTAT® targeting
MELBOURNE, Australia, Jan. 14, 2026 /PRNewswire/ -- Rhythm Biosciences Ltd ('RHY ', the 'Company' or the 'Group') (ASX: RHY), a transformative, predictive cancer diagnostics technology company is pleased to announce the commercial launch of its updated geneTypeTM Colorectal Cancer (CRC) Risk Asses...
Celltrion presents innovative drug pipeline and U.S. manufacturing and R&D expansion strategy at the 44th Annual J.P. Morgan Healthcare Conference
* Celltrion outlines a blueprint for innovative drug development built on its antibody expertise * The company highlights its business strategy to scale U.S. manufacturing and R&D capabilities, strengthening its global supply chain, production and operations INCHEON, South Korea, Jan. 13, 202...
DNV SUPPORTS NOUL IN IVDR CERTIFICATION FOR AI-BASED MEDICAL DIAGNOSTIC PRODUCTS
SEOUL, South Korea, Jan. 14, 2026 /PRNewswire/ -- DNV, the independent global assurance and risk management provider, is partnering with Korean AI-driven blood and cancer diagnostics company Noul to certify their malaria diagnosis, blood cell morphology and cervical cancer testing solutions, under...
Rakuten Medical and LOTTE Biologics Sign Manufacturing Agreement to Support Biopharmaceuticals in Global Oncology Program
SEOUL, South Korea, Jan. 14, 2026 /PRNewswire/ -- Rakuten Medical, Inc. and LOTTE Biologics today announced that they have signed a biopharmaceutical contract manufacturing agreement during the J.P. Morgan Healthcare Conference in San Francisco to strengthen Rakuten Medical's production capabili...
ArisGlobal Accelerates Growth and AI Adoption Following Strong 2025 Performance
More than 100 new and expanded LifeSphere customers, 34 global go-lives, and
rapid enterprise adoption of NavaX signal strong momentum
BOSTON, Jan. 14, 2026 /PRNewswire/ -- ArisGloba
Ractigen Therapeutics Announces First Patient Dosed in Phase II Clinical Trial of RAG-17 for SOD1-ALS
NANTONG, China, Jan. 13, 2026 /PRNewswire/ -- Ractigen Therapeutics is pleased to announce that the first patient has been dosed in the Phase II clinical trial of RAG-17, an innovative siRNA therapy targeting SOD1-mutated amyotrophic lateral sclerosis (ALS). The initial dosing occurred at Second ...
Leo International Group Relocates Global Headquarters to Singapore Ahead of Its Centennial
Embodying a New Model of Inter-generational Enterprise Anchored in Healthcare,
Finance and Education
SINGAPORE, Jan. 13, 2026 /PRNewswire/ -- As Leo International Group
Immunofoco Presents Phase I/IIa Data of IMC002 at ASCO GI 2026, Highlighting a Durable Complete Response Beyond One Year and a 66.7% ORR in Advanced GC/GEJ
SHANGHAI, Jan. 13, 2026 /PRNewswire/ -- Immunofoco, a clinical-stage biotechnology company advancing innovative CAR-T cell therapies for solid tumors, recently announced clinical data from its Phase I/IIa study of IMC002, a VHH-based anti-CLDN18.2 CAR-T therapy, in patients with advanced gastric ...
ER-VIPE Simulation Game Builds Medical Students' Soft Skillsfor Real-World Emergency Care
BANGKOK, Jan. 13, 2026 /PRNewswire/ -- Chulalongkorn University
WuXi Biologics Obtains GMP Certification from UK MHRA for Commercial Manufacturing of an Ophthalmic Biologic
WUXI, China, Jan. 12, 2026 /PRNewswire/ -- WuXi Biologics (2269.HK), a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), announced that two of its manufacturing facilities in Wuxi –Drug Product Facility 5 (DP5) and Drug Product Packaging Center (DPPC) – have r...
WuXi Biologics Obtains GMP Certification from UK MHRA for Commercial Manufacturing of an Ophthalmic Biologic
WUXI, China, Jan. 12, 2026 /PRNewswire/ -- WuXi Biologics (2269.HK), a leading global Contract Research, Development, and Manufacturing Organization (CRDMO), announced that two of its manufacturing facilities in Wuxi –Drug Product Facility 5 (DP5) and Drug Product Packaging Center (DPPC) – have r...
Abbisko Therapeutics Announces FDA Acceptance of the NDA for Pimicotinib for the Treatment of Tenosynovial Giant Cell Tumor
SHANGHAI, Jan. 12, 2026 /PRNewswire/ -- 13 January 2026 (Beijing Time), Abbisko Therapeutics Co., Ltd. ("Abbisko Therapeutics" hereafter, HKEX code: 02256.HK) announcedtoday that the New Drug Application (NDA) for its novel, orally administered, highly selective, and potent small-molecule colony-...
Abbisko Therapeutics Announces FDA Acceptance of the NDA for Pimicotinib for the Treatment of Tenosynovial Giant Cell Tumor
SHANGHAI, Jan. 12, 2026 /PRNewswire/ -- 13 January 2026 (Beijing Time), Abbisko Therapeutics Co., Ltd. ("Abbisko Therapeutics" hereafter, HKEX code: 02256.HK) announcedtoday that the New Drug Application (NDA) for its novel, orally administered, highly selective, and potent small-molecule colony-...
AbbVie and Trump Administration Reach Agreement to Improve Access and Affordability for Americans
* AbbVie will provide low prices in Medicaid, and expand affordable, direct-to-patient offerings for treatments used by millions of Americans through TrumpRx * AbbVie will commit $100 billion in U.S. research and development (R&D) and capital investments, including manufacturing, over the nex...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 299 media titles]
2026-02-09 10:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 299 media titles]
2026-02-10 13:31Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 295 media titles]
2026-02-11 11:00