Medical/Pharmaceuticals
Molecular Stethoscope, Inc. announces new investment from the Alzheimer's Drug Discovery Foundation
Investment by the ADDF will accelerate the translation of Molecular Stethoscope's cf-mRNA liquid biopsy technology platform into products and services for the diagnosis and management of Alzheimer's disease SOUTH SAN FRANCISCO, Calif., June 7, 2022 /PRNewswire/ -- Molecular Stethoscope, a leadin...
Chordia Therapeutics Announces Interim Results of the Phase 1 Clinical Trial of CLK Inhibitor CTX-712 at the 2022 ASCO Annual Meeting
KANAGAWA, Japan, June 7, 2022 /PRNewswire/ -- Chordia Therapeutics Inc. ("Chordia"), a biotech company engaged in the research and development of novel therapies for cancers, today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712, a selective pan-CDC-...
Chordia Therapeutics Announces Interim Results of the Phase 1 Clinical Trial of CLK Inhibitor CTX-712 at the 2022 ASCO Annual Meeting
KANAGAWA, Japan, June 7, 2022 /PRNewswire/ -- Chordia Therapeutics Inc. ("Chordia"), a biotech company engaged in the research and development of novel therapies for cancers, today announced that it has presented the interim results from the Phase 1 clinical trial of CTX-712, a selective pan-CDC-...
SCG Cell Therapy And A*STAR's IMCB Collaborate To Accelerate Clinical Translation of Immune Cell-based Therapy
* The collaboration agreement works toward accelerating the study of SCG's immunotherapy pipeline and candidates such as CAR-T, TCR-T therapies, antibodies, and vaccines. * SCG will contribute its proprietary technologies to the therapeutic development of immune cell-based therapy candidates....
Antengene Announces HREC Approval in Australia for the Phase I Trial of the Small Molecule ATR Inhibitor ATG-018
– Discovered in-house by the internal R&D Team at Antengene, ATG-018 is an orally-bioavailable, small molecule ataxia telangiectasia and Rad3-associated (ATR) kinase inhibitor that targets the DNA damage response (DDR) pathway. – This Phase I study will evaluate the safety, pharmacology and prelim...
ASCO 2022 | Ascentage Pharma Releases Updated Results Demonstrating the Therapeutic Potential of Alrizomadlin (APG-115) plus Pembrolizumab in Patients with Solid Tumors who Progressed on Immunotherapies
SUZHOU, China, and ROCKVILLE, Md., June 6, 2022 /PRNewswire/ -- scentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the updated results from a Pha...
ASCO 2022 | The First Dataset of Olverembatinib (HQP1351) in Patients with GIST Demonstrates Therapeutic Potential with a Clinical Benefit Rate of 83.3%
SUZHOU, China, and ROCKVILLE, Md., June 6, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released the latest results from a Pha...
Pharmactive Enters Strategic Partnership with Nutraconnect for APAC
Pharmactive targets Asia Pacific Market with new collaboration
MADRID, June 7, 2022 /PRNewswire/ -- Pharmactive Biotech Products
iNtRON Completes GLP-TOX Studies of BAL200
* A novel drug candidate for anthrax received orphan drug designation (ODD) by the US FDA. BOSTON and SEOUL, Korea, June 6, 2022 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON" or "Company") announced today that the company has successfully completed the GLP toxicology studies of BAL200. The co...
CStone presents pre-specified overall survival data of sugemalimab for first-line treatment of stage IV non-small cell lung cancer at ASCO 2022
* Sugemalimab is the world's first PD-L1 monoclonal antibody to show the statistically significant and clinically meaningful overall survival improvement in combination with chemotherapy for patients with treatment-naïve stage IV non-small cell lung cancer across both squamous and non-squamous ...
Innovent and AnHeart Jointly Present Updated Phase 2 Efficacy and Safety Data of Taletrectinib (ROS1-TKI) at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting
* In 67 ROS1 TKI-naïve patients, the confirmed objective response rate (cORR) and disease control rate (DCR) were 92.5% and 95.5%, respectively. * In 38 crizotinib-pretreated patients, the cORR and DCR were 50% and 78.9%, respectively. * In 12 patients with brain metastasis and measurable...
Telix Showcasing Innovation in Theranostics at SNMMI
MELBOURNE, Australia and INDIANAPOLIS, June 7, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces its presence at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting to be held inVancouver from June 11-14. Gallium-based pr...
MGI Announces Commercial Availability of DNBSEQ™ Sequencers* in the United States
SAN JOSE, Calif., June 6, 2022 /PRNewswire/ -- MGI Americas (MGI), today announced that its innovative CoolMPS sequencing chemistry and instruments* will become commercially available inthe United States beginning from August 29,2022. More details about the launch will be revealed at the 22nd ...
Menarini Group and Radius Health, Inc. present a subgroup analysis from the elacestrant pivotal phase 3 EMERALD clinical trial at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting
Subgroup analysis of patients with no prior chemotherapy in EMERALD: A phase 3 trial evaluating elacestrant, an investigational oral selective estrogen receptor degrader (SERD), vs. investigator's choice of endocrine monotherapy for ER+/HER2- advanced/metastatic breast cancer (mBC) FLORENCE, Ita...
Prenetics Announces First Quarter 2022 Financial Results After a Successful Listing on the Nasdaq under the Ticker "PRE"
LONDON and HONG KONG, June 6, 2022 /PRNewswire/ -- Prenetics Global Limited (NASDAQ: PRE) ("Prenetics" or the "Company"), a global leader in genomic and diagnostic testing, today announced its unaudited financial results for the first quarter endedMarch 31, 2022. The Company recorded strong opera...
IND approval from the US FDA for Phase II SAR-Bombesin imaging trial in prostate cancer
Highlights * IND approval received for SAR-Bombesin product, enabling a Phase II "SABRE" imaging trial to detect prostate cancer in up to 50 PSMA-negative participants in the US * Approximately 20% of prostate cancer patients with biochemical recurrence (BCR) are PSMA-PET negative and theref...
Insilico Medicine Raises $60 Million in Series D Financing to Advance Pipeline and Launch AI-powered Drug Discovery Robotics Laboratory
NEW YORK, June 6, 2022 /PRNewswire/ -- Insilico Medicine, a clinical-stage end-to-end artificial intelligence (AI)-driven drug discovery company, announced today that it has completed a$60 million Series D financing from a syndicate of global investors with expertise in investing in the biopharm...
Insilico Medicine Raises $60 Million in Series D Financing to Advance Pipeline and Launch AI-powered Drug Discovery Robotics Laboratory
NEW YORK, June 6, 2022 /PRNewswire/ -- Insilico Medicine, a clinical-stage end-to-end artificial intelligence (AI)-driven drug discovery company, announced today that it has completed a$60 million Series D financing from a syndicate of global investors with expertise in investing in the biopharm...
Senhwa's Pindnarulex in Combination Study with Pfizer's Talazoparib for the Treatment of Prostate Cancer Granted Approval to Initiate from Australian HREC
TAIPEI and SAN DIEGO, June 6, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that it has received written approval from the Human Research Ethics Committ...
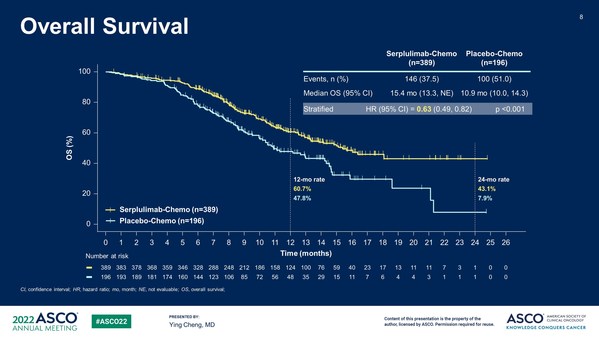
ASTRUM-005: Henlius Released Phase 3 Study Results for the First-line Treatment of Small Cell Lung Cancer of Serplulimab at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that an international randomized phase 3 study (ASTRUM-005) of HANSIZHUANG (serplulimab), an anti-PD-1 mAb independently developed by Henlius, as first-line treatment for extensive-stage small-cell lung can...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 273 media titles]
2024-04-24 17:09Introducing Wacom Movink: The first OLED pen display, and the thinnest and lightest Wacom pen display ever
[Picked up by 268 media titles]
2024-04-24 13:00