Medical/Pharmaceuticals
CARsgen Therapeutics Presents Updated Data for CT041 Claudin18.2 CAR T-cells in Solid Tumors at ASCO 2022
SHANGHAI, June 6, 2022 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that at the 2022 American Society of Clinical Oncology (ASCO) Annual M...
Medison Pharma Announces Multi-Regional Partnership Agreement with argenx to Commercialize Efgartigimod Across Europe and Israel
PETACH TIKVAH, Israel, June 6, 2022 /PRNewswire/ -- Medison Pharma
NeuroAiD has shown favourable outcome in patients suffering from spinal cord injuries
* SATURN study aimed at assessing the safety and effects of a 6-month course of NeuroAiD (MLC601/MLC901) to augment functional and motor recovery after severe spinal cord injury (SCI). * NeuroAiD was safe as add-on treatment in patients with severe SCI. * One third of patients improved on AS...
CPHI China opens for international partnering after China rises in CPHI Index
The CPHI China Hosted Buyer Programme is empowering new partnering in China
SHANGHAI, June 6, 2022 /PRNewswire/ -- CPHI & P-MEC China
CStone and Pfizer announce NMPA approval of sugemalimab in patients with unresectable stage III non-small cell lung cancer
* The National Medical Products Administration approved sugemalimab for the treatment of patients with unresectable stage III non-small cell lung cancer whose disease has not progressed following concurrent or sequential platinum-based chemoradiotherapy * Sugemalimab became the first anti-PD-...
CStone and Pfizer announce NMPA approval of sugemalimab in patients with unresectable stage III non-small cell lung cancer
* The National Medical Products Administration approved sugemalimab for the treatment of patients with unresectable stage III non-small cell lung cancer whose disease has not progressed following concurrent or sequential platinum-based chemoradiotherapy * Sugemalimab became the first anti-PD-...
GILEAD SCIENCES APPOINTS DUSTIN HAINES AS NEW LEADER FOR ASIA 5 REGION
-- Mr Haines Brings a Strong Track Record of Global Commercial Successes and Corporate Leadership with a Career that Spans 20 Years in Pharmaceuticals -- -- He Joins Gilead's Asia 5 Team as the Company Celebrates 10 Years of Presence inAsia -- HONG KONG, June 6, 2022 /PRNewswire/ -- Gilead Scien...
Poster Highlighting Phase II Clinical Study of KN026 for HER2-expressing Advanced GC/GEJ Presented at 2022 ASCO
SUZHOU, China, June 6, 2022 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) and CSPC Pharmaceutical Group Co., Ltd. (stock code: 1093.HK) jointly announced that data from a phase II clinical study of KN026 (HER2 bispecific antibody) monotherapy in patients with previously treated, advance...
Research Updates on KN046 Presented at 2022 ASCO
SUZHOU, China, June 6, 2022 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that research updates from four clinical studies of KN046 (PD-L1/CTLA-4 bispecific antibody) presented as a poster or published online at the 2022 American Society of Clinical Oncology Annual Meeting (20...
Akeso releases promising data of Ivonescimab (PD-1/VEGF BsAbs, AK112) for advanced NSCLC at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released clinical details in poster presentation featuring phase Ib...
Akeso announces oral presentation featuring promising clinical data of Cadonilimab (PD-1/CTLA-4 BsAbs, AK104) for the first-line treatment of R/M cervical cancer at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released updated results of Cadonilimab (PD-1/CTLA-4 Bispecific, AK...
Akeso releases promising data of Ivonescimab (PD-1/VEGF BsAbs, AK112) combined with chemotherapy in advanced NSCLC at ASCO 2022
HONG KONG, June 5, 2022 /PRNewswire/ -- Akeso, Inc. (9926.HK) ( "Akeso" ), a China-based biopharmaceutical company focusing on the development and commercialization of innovative therapeutic antibodies for Oncology & Immunology, released clinical details in poster discussion featuring a phase II ...
WuXi Biologics Launches First Commercial Drug Product Facility for Pre-Filled Syringes
* WuXi Biologics offers global partners high-efficiency and high-quality manufacturing services for clinical trial and commercial supply by increasing pre-filled syringes (PFS) capacity to 17 million units per year. * WuXi Biologics has established a global manufacturing network with 9 drug p...
Innovent Presents Clinical Data of IBI110 (Anti-LAG-3 Monoclonal Antibody) at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting
SAN FRANCISCO and SUZHOU, China, June 6, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and ...
Innovent and IASO Bio Jointly Announce the NMPA Acceptance of the New Drug Application for Equecabtagene Autoleucel for the Treatment of Relapsed and/or Refractory Multiple Myeloma
SAN FRANCISCO and SUZHOU, China, June 6, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and...
Innovent Presents Clinical Data of Phase I Study for IBI351 (KRAS G12C Inhibitor) as Monotherapy for Solid Tumors at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting
SAN FRANCISCO and SUZHOU, China, June 6, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and ...
CStone presents updated results of anti-PD-1 antibody nofazinlimab in combination with lenvatinib as first-line treatment in patients with unresectable hepatocellular carcinoma (HCC) at ASCO 2022
* Nofazinlimab in combination with lenvatinib as first-line treatment for unresectable hepatocellular carcinoma demonstrated an objective response rate of 45.0%. The median duration of response was not yet reached as of the data cutoff date (4.2 to 18.7+ months). The median progression free sur...
Everest Medicines' Licensing Partner Gilead Sciences Announces Positive Results from Phase 3 TROPiCS-02 Study of Trodelvy® in Heavily Pre-treated HR+/HER2- Metastatic Breast Cancer Patients
FOSTER CITY, Calif. and SHANGHAI, June 4, 2022 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK) announces today that its licensing partner, Gilead Sciences, Inc. (Nasdaq: GILD), reported positive results from the primary analysis of the Phase 3 TROPiCS-02 study of Trodelvy® (sacituzumab govitecan...
CStone presents clinical results of sugemalimab in patients with relapsed or refractory extranodal natural killer/T-cell lymphoma via an oral abstract session at ASCO 2022
* GEMSTONE-201 is the largest registrational clinical study of an anti-PD-(L)1 antibody reported so far in patients with relapsed or refractory extranodal natural killer/T-cell lymphoma (R/R ENKTL). * Results on the primary endpoint showed that sugemalimab significantly improved objective res...
Gracell Biotechnologies Schedules Clinical Update Call After EHA2022
SAN DIEGO, Calif. and SUZHOU and SHANGHAI, China, June. 3, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, today a...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
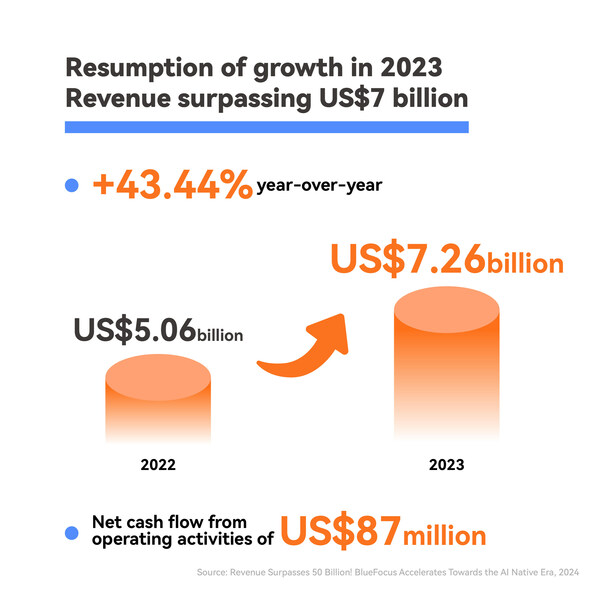
2024-04-22 06:00Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 273 media titles]
2024-04-24 17:09Introducing Wacom Movink: The first OLED pen display, and the thinnest and lightest Wacom pen display ever
[Picked up by 268 media titles]
2024-04-24 13:00