Pharmaceuticals
ImmunoForge Receives IND Approval for Phase 2 Clinical Trial of 'Pemziviptadil' for DMD Cardiomyopathy Treatment from FDA, and KF1601, a chronic myeloid leukemia treatment, phase 1 clinical trial IND approved by MFDS
SEOUL, South Korea, Jan. 14, 2025 /PRNewswire/ -- ImmunoForge announced that it has received approval for the Phase 2 clinical trial IND for 'Pemziviptadil (development code name PF1804),' a treatment for DMD (Duchenne Muscular Dystrophy) cardiomyopathy, from the U.S. Food and Drug Administration...
Telix Presentation to the 43rd Annual J.P. Morgan Healthcare Conference
MELBOURNE, Australia and INDIANAPOLIS, Jan. 14, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today advises that Dr.Christian Behrenbruch, Managing Director and Group CEO, will be presenting this week at the 43rd Annual J.P. Morgan Healthcare Confe...
BioCina and NovaCina Merger Bolsters Global CDMO Industry
ADELAIDE, South Australia and PERTH, Western Australia, Jan. 14, 2025 /PRNewswire/ -- Global Contract Development and Manufacturing Organizations (CDMOs) BioCina and NovaCina announced a strategic merger that will create a powerful brand in biopharmaceutical and small molecule contract manufactur...
Simcere Zaiming and AbbVie Announce Partnership to Develop a Novel Trispecific Antibody Candidate in Multiple Myeloma
SHANGHAI and NORTH CHICAGO, Ill., Jan. 13, 2025 /PRNewswire/ -- Simcere Zaiming, a subsidiary of Simcere Pharmaceutical Group Ltd (HKEX: 2096) and AbbVie Inc. (NYSE: ABBV) today announced an option to license agreement to develop SIM0500, an investigational new drug candidate. SIM0500 is currentl...
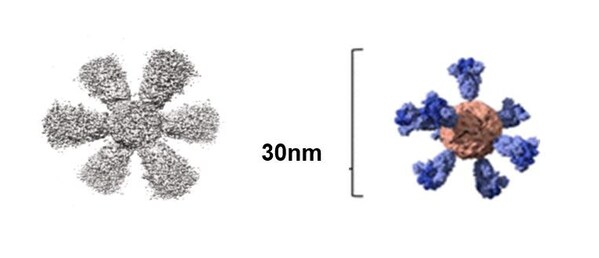
Dx&Vx Presents a New Paradigm for Next-Generation Infectious Disease Response with the Development of a Universal Vaccine
- Preparing for Phase 2 Global Trials of a Ferritin Platform-Based Virus-Like Particle Universal Coronavirus Vaccine - Based on the Excellent Safety and Immune Response in Phase 1 Clinical Results, with a Plan to Extend Administration Routes and Indications SEOUL, South Korea, Jan. 13, 2025 /PRNe...
CARsgen's Allogeneic CD19/CD20 CAR-T Therapy Administers First Dose in an Investigator-Initiated Trial
SHANGHAI, Jan. 13, 2025 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that KJ-C2219, an allogeneic CAR T-cell therapy targeting CD19/CD20, ...
CBC Group's R-Bridge Announces US$40M Synthetic Royalty-Backed Financing for Mirxes to Advance Global Expansion
- R-Bridge will provide bespoke financing of US$40 million with a synthetic royalty structure and flexible repayment terms - Financing will accelerate Mirxes, Singapore -based miRNA tech company's innovation and commercialization of cancer early detection solutions in new markets - Transacti...
Telix Exceeds FY24 Guidance with US$142M Q4 Revenue
MELBOURNE, Australia and INDIANAPOLIS, Jan. 13, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today provides an update on its commercial and operational performance for the quarter ended31 December 2024 (Q4 2024). Sustained revenue growth * Q...
Scintimun® Commercialization Partnership with Curium Pharma
MELBOURNE, Australia and LIÈGE, Belgium, Jan. 13, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX; Nasdaq: TLX, Telix, the Company) today announces that it has entered into an agreement with Curium Pharma for the transfer of marketing and distribution rights for Scintimun® (99m Tc-be...
WuXi AppTec Joins the Pharmaceutical Supply Chain Initiative (PSCI)
SHANGHAI, Jan. 12, 2025 /PRNewswire/ -- WuXi AppTec, a global company that provides a broad portfolio of R&D and manufacturing services to enable companies in the pharmaceutical and life sciences industries, announced that it has joined the Pharmaceutical Supply Chain Initiative (PSCI) as a Suppl...
Telix to Acquire Next-Generation Therapeutic Assets and Innovative Biologics Technology Platform
MELBOURNE, Australia and INDIANAPOLIS, Jan. 13, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Nasdaq: TLX, Telix, the Company) today announces it has entered into an asset purchase agreement with antibody engineering company ImaginAb, Inc. (ImaginAb) to acquire a pipeline of next...
Kelun-Biotech Announce Exclusive License Agreement For SKB378/HBM9378, an Anti-thymic Stromal Lymphopoietin (TSLP) Monoclonal Antibody (mAb).
CHENGDU, China, Jan. 11, 2025 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. (the "Company") announced that the Company and HBM Holdings Limited ("Harbour BioMed") (together with the Company, the "Licensors"), have entered into an exclusive license agreement (the "License Agr...
Regor Releases Phase 2a Topline Results for RGT-075, an Oral Once-daily Small Molecule GLP-1R Agonist, and Begins Phase 2b Study in the US for the Treatment of Obesity
* Regor announced its 12-week single-dose Phase 2a trial results for RGT-075, a once-daily oral GLP-1RA, reporting 5% placebo-adjusted weight-loss with no plateau * No treatment-related severe adverse events (AEs), with only 4% discontinuation rate due to AEs, same as placebo * Enrollment o...
Menarini Group and Insilico Medicine Enter a Second Exclusive Global License Agreement for an AI Discovered Preclinical Asset Targeting High Unmet Needs in Oncology
* This is the second asset Menarini Group has inlicensed from Insilico Medicine which was discovered through their generative AI platform, similar to the preclinical stage KAT6 inhibitor (MEN2312) licensed a year ago and which advanced rapidly into the clinical phase. * Under the agreement, M...
Menarini Group and Insilico Medicine Enter a Second Exclusive Global License Agreement for an AI Discovered Preclinical Asset Targeting High Unmet Needs in Oncology
* This is the second asset Menarini Group has inlicensed from Insilico Medicine which was discovered through their generative AI platform, similar to the preclinical stage KAT6 inhibitor (MEN2312) licensed a year ago and which advanced rapidly into the clinical phase. * Under the agreement, M...
Harbour BioMed Announces License Agreement with Windward Bio for HBM9378/SKB378, an Anti-TSLP Fully Human Antibody for Immunological Diseases
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SHANGHAI, Jan. 10, 2025 /PRNewswire/ -- Harbour BioMed (HKEX: 02142, the "Company"), a global biopharmaceutical company committed to the discovery, development and commercialization of novel antibody therapeutics focusing on immune-oncology and immuno...
JW Therapeutics Announces Receipt of Breakthrough Therapy Designation for Carteyva® in China as Second-line Treatment in Relapsed or Refractory adult Large B-cell Lymphoma
SHANGHAI, Jan. 10, 2025 /PRNewswire/ -- JW Therapeutics (HKEx: 2126), an independent and innovative biotechnology company focused on developing, manufacturing and commercializing cell immunotherapy products, announced that the Center for Drug Evaluation (CDE) of the National Medical Products Adm...
Sciwind Biosciences Announces Global Licensing and Collaboration Agreement for Metabolic Disease Portfolio
HANGZHOU, China, Jan. 10, 2025 /PRNewswire/ -- Hangzhou Sciwind Biosciences Co., a pre-commercial biopharmaceutical company focusing on developing innovative therapies to treat metabolic diseases, today announced a licensing and collaboration agreement for the global development and commercializa...
SN BioScience Propels SNB-101 to Phase 2 with IND Clearance from FDA
SEOUL, South Korea, Jan. 10, 2025 /PRNewswire/ -- SN BioScience today announced that its lead asset SNB-101 has received Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration (FDA) for a Phase1b/2 clinical trial. This achievement follows the designation of SNB-101 as...
In 2024, Complete Genomics expanded U.S. manufacturing and technology capabilities toward a diversified, reliable supply chain for its flexible and cost-effective NGS solutions
SAN JOSE, Calif., Jan. 9, 2025 /PRNewswire/ -- In 2024, Complete Genomics, a pioneering genomic sequencing company, expanded its U.S. manufacturing footprint with augmented technology capabilities to diversify its supply chain helping it to continue reliably serving customers with flexible, cost-...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Rockwell Automation Strengthens Industrial Cybersecurity with New Security Operations Center in Singapore
[Picked up by 299 media titles]
2026-02-09 10:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 299 media titles]
2026-02-10 13:31Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 296 media titles]
2026-02-11 11:00