Pharmaceuticals
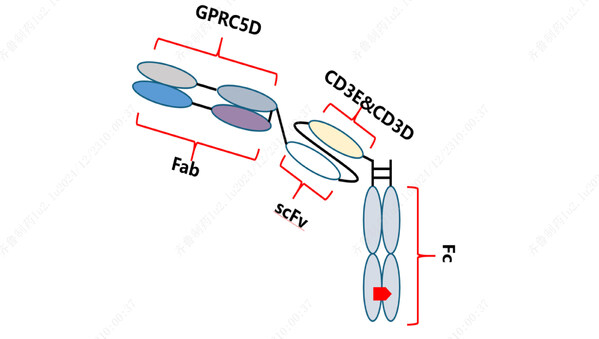
Preliminary Results of Qilu Pharmaceutical's Phase Ia Study on GPRC5D/CD3-Targeting Bispecific Antibody QLS32015 Unveiled at 2024 ASH Annual Meeting
JINAN, China, Dec. 25, 2024 /PRNewswire/ -- During the 66th Annual Meeting of the American Society of Hematology (ASH) held inSan Diego from December 7 to 10 , preliminary results from the first human trial of QLS32015, a novel anticancer drug developed by Qilu Pharmaceutical for treating relapse...
Ractigen Therapeutics Announces First Patient Dosed in Phase I Clinical Trial for RAG-17 in SOD1-ALS
NANTONG and SUZHOU, China, Dec. 24, 2024 /PRNewswire/ -- Ractigen Therapeutics, a clinical-stage pharmaceutical company dedicated to developing RNA-based innovative therapies, today announced the successful dosing of the first patient in the Phase I clinical trial of RAG-17, an innovative siRNA t...
Keymed Biosciences Announces Approval Of Stapokibart For The Treatment Of Chronic Rhinosinusitis With Nasal Polyposis
CHENGDU, China, Dec. 23, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced the National Medical Products Administration (the "NMPA") ofChina has recently approved the supplemental New Drug Application (the "sNDA") of Stapokibart (anti-IL-4Rα monoclonal antibody, trade nam...
Porton Pharma Solutions and Dragon Sail Pharmaceutical Form Strategic Partnership to Build a New Ecosystem for ADC Drug Development, Production, and Supply Chain
CHONGQING, China, Dec. 23, 2024 /PRNewswire/ -- On December 20, 2024, Porton Pharma Solutions (hereinafter referred to as "company" or "Porton") and Dragon Sail Pharmaceutical (Shanghai) Co., Ltd. (a subsidiary of Guilin Sanjin, hereinafter referred to as "Dragon Sail Pharmaceutical") formally si...
Kickstart 2025 with Expert Holiday Health Tips
KUALA LUMPUR, Malaysia, Dec. 23, 2024 /PRNewswire/ -- As Pantai Hospital Kuala Lumpur (PHKL) celebrates 50 years of excellence in patient care, it's a moment to reflect on how small, proactive steps can make a significant difference to your health. The holiday season is a time of joy, but it also...
Aptamer developed by HKBU for treating rare bone disease 'X-linked hypophosphatemia' receives Orphan Drug Designation and Rare Pediatric Disease Designation by U.S. FDA
HONG KONG, Dec. 23, 2024 /PRNewswire/ -- A research led by Hong Kong Baptist University (HKBU) and the Shanghai Sixth People's Hospital Affiliated to School of Medicine at Shanghai Jiao Tong University (Shanghai Sixth People's Hospital) has discovered that an aptamer developed by HKBU can be used...
SCG Cell Therapy partners A*STAR to accelerate RNA-based therapeutic development in Singapore
* SCG Cell Therapy signed a Memorandum of Understanding (MoU) with A*STAR Bioprocessing Technology Institute (A*STAR BTI) and Nucleic Acid Therapeutics Initiative (NATi) to advance ribonucleic acid (RNA)-based therapeutic manufacturing process development and clinical translation. * The parti...
CStone Announces Submission of Clinical Trial Application in Australia for CS2009, an Innovative PD-1/VEGF/CTLA-4 Trispecific Antibody
SHANGHAI, Dec. 22, 2024 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), an innovation-driven biopharmaceutical company focused on the research and development of anti-cancer therapies, announced today the submission of clinical trial application inAustralia for CS2009 (PD-1/VEGF/CT...
Macrogen Consortium win tender for National Bio Big Data Project
Illumina is proud to be selected as sequencing technology partner to the Macrogen Consortium. SEOUL, South Korea, Dec. 23, 2024 /PRNewswire/ -- Macrogen a global healthcare company that specializes in precision medicine and Illumina Inc. (NASDAQ: ILMN), a global leader in DNA sequencing and arra...
YolTech Therapeutics Announces Successful Completion of Dose Escalation Phase in Phase I Trial of YOLT-201 for ATTR
SHANGHAI, Dec. 21, 2024 /PRNewswire/ -- YolTech Therapeutics today announced updated data from its ongoing Phase I/IIa clinical trial of YOLT-201, a first-in-class CRISPR/Cas9-based in vivo gene-editing therapy for ATTR amyloidosis. The trial has completed dosing in eight participants, including ...
United Imaging Intelligence at RSNA 2024: Empowering a More Intelligent and Connected World with Medical AI
CHICAGO, Dec. 20, 2024 /PRNewswire/ -- As RSNA 2024 concluded, United Imaging Intelligence (UII), a subsidiary of United Imaging Group specializing in medical AI, made a significant impression with a visionary showcase that seamlessly aligned with the event's theme: "Building Intelligent Connecti...
Medicilon Appoints Dr. Lilly Xu as Chief Technology Officer
BOSTON, Dec. 20, 2024 /PRNewswire/ -- Medicilon, a leading preclinical contract research organization (CRO), has named Dr.Lilly Xu as its new Chief Technology Officer (CTO). With over30 years of experience in preclinical drug development, Dr. Xu will lead Medicilon's technological innovation a...
Innovent Receives Approval of DOVBLERON® (Taletrectinib Adipate Capsule, ROS1 Inhibitor) by China's National Medical Products Administration
SAN FRANCISCO and SUZHOU, China, Dec. 20, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune...
LakeShore Biopharma Reports Unaudited Financial Results for the First Half of Fiscal Year 2025 and Updates Full-Year Guidance
* Achieved total revenue of RMB 371.9 million, reflecting a growth of 36.2% YoY * Achieved gross profit of RMB 307.3 million, a growth of 39.1% YoY * Gross margin increased to 82.6% from 80.9% in the same period of FY2024 * Total operating expenses decreased to RMB 276.4 million, down 31.6%...
BGM Group and Jointown Deepen Cooperation, Ushering in a New Era of Pharmaceutical Development
CHENGDU, China, Dec. 19, 2024 /PRNewswire/ -- BGM Group Ltd. (NASDAQ: BGM) ("the Company" or "BGM"), Mr. Xin Zhanchang, Chairman of the Board of Directors of BGM Group and Chairman of Gansu Qilianshan Pharmaceutical Co., Ltd., led the sales team to visit their long-term strategic partner, Jointow...
Telix Manufacturing Solutions, Brussels South Update: Cyclotron Installation Complete
MELBOURNE, Australia and INDIANAPOLIS, Dec. 19, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX; Nasdaq: TLX, Telix, the Company) today announces that it has completed the installation of two new cyclotrons at Telix Manufacturing Solutions (TMS) in Brussels South,Belgium, facilita...
LG Corp. Chairman and CEO Kwang Mo Koo sends 2025 New Year Address to 270,000 global employees on December 19
* "Let's Shape LG's Future with the DNA of Challenge and Change We've had Since Day 1" * Highlighting LG's Spirit – the DNA of challenge and change since Day 1 - "LG's Day 1 spirit of choosing what others wouldn't dare comes from our DNA of challenge and change as we serve our customer...
Fangzhou Inc. and Bristol Myers Squibb China Join Forces on a Strategic Alliance to Advance Internet Healthcare
SHANGHAI, Dec. 18, 2024 /PRNewswire/ -- Fangzhou Inc. ("Fangzhou" or the "Company") (06086.HK), a pioneer in Internet healthcare solutions, announced a strategic alliance with Bristol Myers Squibb ("BMS")China on December 16th. The partnership was officially formalized with the signing of a colla...
Pharus Diagnostics LLC Announces Validation of a Laboratory-Developed Test for Early Pancreatic Cancer Detection
LOS ANGELES, Dec. 19, 2024 /PRNewswire/ -- Pharus Diagnostics LLC. (PharusDx), an innovator in non-invasive diagnostic tests, today announced a significant step forward in the fight against pancreatic cancer. The company is announcing availability of its OncoSweep™ Pancreas Spotlight, a liquid bi...
Clarity expands its pipeline with a novel optimised FAP-targeted radiopharmaceutical
Highlights * Clarity has developed a proprietary fibroblast activation protein (FAP)-targeted radiopharmaceutical product that can be used with the perfect pairing of copper isotopes for the diagnosis and treatment of cancer. * The product, termed SAR-bisFAP, has shown strong tumour targeting...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 318 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 315 media titles]
2026-02-02 10:00Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 309 media titles]
2026-01-27 13:00Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 294 media titles]
2026-01-27 18:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 284 media titles]
2026-02-01 19:35