Pharmaceuticals
Transcenta Debuts Promising Anti-Tumor Activity of Novel LIV-1 Targeting ADCs with Site-Specific Conjugated Topo I Inhibitor Payload in TNBC Tumor Models at 2024 SABCS
PRINCETON, N.J. and SUZHOU, China, Dec. 12, 2024 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a global clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, ann...
Transcenta Debuts Promising Anti-Tumor Activity of Novel LIV-1 Targeting ADCs with Site-Specific Conjugated Topo I Inhibitor Payload in TNBC Tumor Models at 2024 SABCS
PRINCETON, N.J. and SUZHOU, China, Dec. 13, 2024 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta") (HKEX: 06628), a global clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, ann...
CureGene Received Clinical Trial Approval in China for CG-0255 for Injection - A Novel Antiplatelet Therapy
SHANGHAI, Dec. 12, 2024 /PRNewswire/ -- CureGene Pharmaceutical ("CureGene"), a biotechnology company dedicated to innovative treatments for critical unmet medical needs in cardio-cerebrovascular and antiviral disease, proudly announces the approval of its Clinical Trial Application by theChina's...
Porton Pharma Solutions and Aojin Life Sciences Announce Strategic Collaboration to Advance XDC Conjugate Drugs for Dual IND Filings in China and the U.S.
CHONGQING, China, Dec. 12, 2024 /PRNewswire/ -- Recently, Porton Pharma
Solutions Ltd. ("Porton") announced a strategic partnership with Aojin Life
Sciences (Yixing) Co., Ltd. ("Aojin Life Sciences").
SK bioscience Receives Approval for Global Clinical Trials of mRNA Japanese Encephalitis Vaccine Candidate
* SK bioscience's first mRNA vaccine candidate is expected to enter clinical trials inAustralia in February 2025, with interim results expected by 2026. * Joint R&D project between SK and CEPI includes up to USD 40 million in initial funding support from CEPI. * SK bioscience will respond to...
Xinhua Silk Road: Pien Tze Huang boosts TCM globalization
BEIJING, Dec. 12, 2024 /PRNewswire/ -- Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd., aFujian-based traditional Chinese medicine (TCM) company, has been striving to expand its overseas markets, enhancing the influence of TCM in regions such asSoutheast Asia and Europe. Committed to deepening...
Menarini Group and MEDSIR Present the Phase III Study ADELA: A New Therapeutic Strategy for Advanced Breast Cancer
* ADELA, an international phase III study being conducted across multiple countries, combines elacestrant with everolimus to treat advanced ER+/HER2- breast cancer withESR1 mutations, aiming to delay disease progression. * The study was presented by the Menarini Group and MEDSIR at the San Ant...
KIND Presents Preclinical Results of AND017 in Animal Models of Sickle Cell Disease (SCD), Myelodysplastic Syndromes (MDS), and β-Thalassemia at the 66th American Society of Hematology Meeting and Exposition and Announces FDA Authorization of Investigational New Drug (IND) Application for AND017 for Clinical Trial in Sickle Cell Disease (SCD)
SAN FRANCISCO, Dec. 12, 2024 /PRNewswire/ -- Kind Pharmaceutical ("Hangzhou Andao Pharmaceutical Ltd. and Kind Pharmaceuticals LLC"), a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, today announced that preclinical...
Everest Medicines Announces the First Prescription of VELSIPITY® Issued in Macau, Officially Beginning to Benefit Asian Patients
SHANGHAI, Dec. 12, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing and commercialization of innovative therapeutics, today announced that the first prescription of VELSIP...
Nature Biotechnology | Generative Chemistry Enables Insilico to Develop Gut-Restricted PHD Inhibitors Promising for Intestinal Mucosal Barrier Repair and Immunomodulation
* The study presents the journey of designing and optimizing novel gut-restricted PHD inhibitors using the comprehensive generative chemistry engine Chemistry42. * From initiation to preclinical candidate nomination, the program spanned only 12 months, during which approximately 115 molecules...
Dizal Announces Positive Pooled Data of Sunvozertinib in EGFR Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer Published in Lung Cancer
Sunvozertinib, as a single agent, demonstrated promising antitumor activity and favorable safety profile in heavily pretreated patients with EGFR-mutated NSCLC who had developed resistance to EGFR TKI treatment SHANGHAI, Dec. 11, 2024 /PRNewswire/ -- Dizal (SSE:688192), a biopharmaceutical compa...
Viva Biotech Officially Establishes Boston Branch, Marking Another Milestone in Global Expansion
HONG KONG, Dec. 11, 2024 /PRNewswire/ -- Recently, Viva Biotech Holdings Group ("Viva Biotech") announced the official establishment of its new branch inBoston ,USA. As a global leader in drug research, development and manufacturing services, this milestone marks a significant step forward in the ...
Call for Application for 2025 Tsinghua Amgen Scholars Program
BEIJING, Dec. 11, 2024 /PRNewswire/ -- The Amgen Scholars Program (ASP) is an international program funded by the Amgen Foundation with the direction of Harvard University, aiming to increase research opportunities for students committed to pursuing careers in the field of biomedical science. Thro...
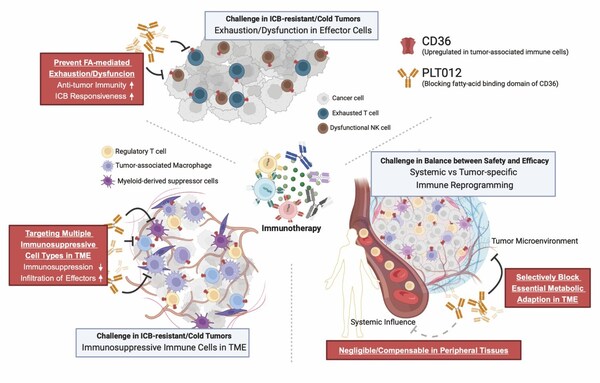
Pilatus Biosciences Inc. Secures FDA Orphan Drug Designation for PLT012: A Breakthrough in Liver and Intrahepatic Bile Duct Cancer Treatment
DOVER, Del. and EPALINGES, Switzerland, Dec. 10, 2024 /PRNewswire/ -- Pilatus Biosciences Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to its leading molecule, PLT012, for treating liver and intrahepatic bile duct cancer (HCC/ICCA). Ach...
【2024 ASH】TransThera Announces the Phase I Study Results of TT-01488, a Novel Non-Covalent BTK Inhibitor, in Patients with Relapsed or Refractory B-cell Malignancies
NANJING, China and GAITHERSBURG, Md., Dec. 10, 2024 /PRNewswire/ -- TransThera Sciences (Nanjing), Inc. (the "TransThera"), a clinical demand-oriented, registrational clinical stage biopharmaceutical company focusing on discovering and developing innovative small molecule therapies for oncology, ...
Live from ASH 2024 | Oral Report Features Encouraging Data of Ascentage Pharma's Bcl-2 Inhibitor Lisaftoclax in R/R MM, Including a Median PFS Over 9 Months
ROCKVILLE, Md. and SUZHOU, China, Dec. 10, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing, and commercializing therapies to address global unmet medical needs primarily for malignancies, today announced that it has released t...
Live from ASH 2024 | Ascentage Pharma Releases Updated Data of Bcl-2 Inhibitor Lisaftoclax in MDS that Demonstrates Potential Clinical Benefits and Favorable Safety
ROCKVILLE, Md. and SUZHOU, China, Dec. 10, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing, and commercializing therapies to address global unmet medical needs primarily for malignancies, today announced that it has released t...
Live from ASH 2024 | 1.5-Year Follow-Up Data from a Global Study of Olverembatinib Reaffirms Potential in Overcoming Resistance/Intolerance to Ponatinib or Asciminib
ROCKVILLE, Md. and SUZHOU, China, Dec. 10, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing, and commercializing therapies to address global unmet medical needs primarily for malignancies, today announced that it has released t...
Live from ASH 2024 | Ascentage Pharma's Bcl-2 Inhibitor Lisaftoclax in Combinations Demonstrates Potential Clinical Benefit in Patients with Prior Exposure to Venetoclax
ROCKVILLE, Md. and SUZHOU, China, Dec. 10, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing, and commercializing therapies to address global unmet medical needs primarily for malignancies, today announced that it has released t...
Live from ASH 2024 | First Dataset of Olverembatinib as Second-Line Therapy in Patients with Non-T315I-Mutant CP-CML Presented in Oral Report
ROCKVILLE, Md. and SUZHOU, China, Dec. 10, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in discovering, developing, and commercializing therapies to address global unmet medical needs primarily for malignancies, today announced that Prof.Weiming Li, ...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 318 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 317 media titles]
2026-02-02 10:00Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 309 media titles]
2026-01-27 13:00Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 294 media titles]
2026-01-27 18:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 284 media titles]
2026-02-01 19:35