Pharmaceuticals
Allorion Therapeutics Announces Exclusive Option and Global License Agreement for Novel Preclinical-Stage EGFR L858R Allosteric Inhibitor Program with AstraZeneca
NATICK, Mass., Jan. 2, 2024 /PRNewswire/ -- Allorion Therapeutics ("Allorion"), a US andChina-based biotechnology company that focuses on the discovery of new small molecule drugs for treating cancer and autoimmune diseases, has entered into an exclusive option and global license agreement with A...
Gannex Announces Positive Interim Results from 52-Week Phase II Clinical Trial of Once-Daily ASC41 Tablet in Patients with Biopsy-Confirmed Non-alcoholic Steatohepatitis
--Up to 68.2% mean relative reduction in liver fat content from baseline in biopsy-confirmed non-alcoholic steatohepatitis (NASH) patients receiving 12-week treatment of ASC41 tablet --At Week 12, up to 93.3% patients achieved at least a 30% relative reduction in liver fat content from baseline ...
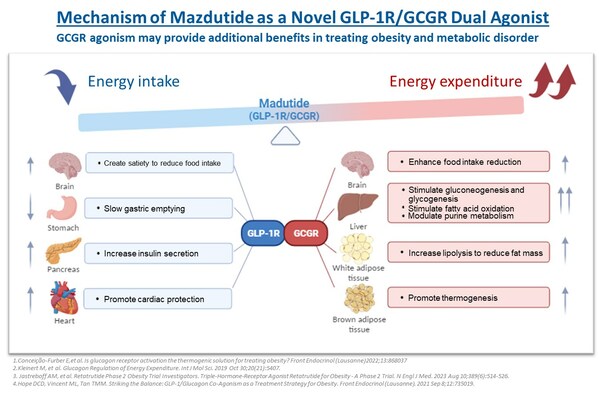
Innovent Dosed First Participant in Phase 3 Clinical Study (GLORY-2) of Mazdutide (IBI362) Higher Dose 9 mg in Chinese Adults with Obesity
ROCKVILLE, Md. and SUZHOU, China, Jan. 2, 2024 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and o...
ANAESTHESIOLOGIST HERO, DOCTOR'S QUICK THINKING SAVES CYCLIST'S LIFE DURING LEKAS NIGHT RIDE 2023
Off-duty doctor's decisive action and collaboration with fellow physician-cyclists lead to a successful resuscitation and life-saving measures. SELANGOR, MALAYSIA, Dec. 29, 2023 /PRNewswire/ -- In an extraordinary display of quick thinking and medical expertise, Dr. Shahridan Bin Mohd Fathil, an ...
Positive Results In The Interim Analysis Of The Clinical Trial Of Recbio's Novel Adjuvanted Recombinant Shingles Vaccine REC610 In The Philippines
TAIZHOU, China, Dec. 29, 2023 /PRNewswire/ -- Jiangsu Recbio Technology Co., Ltd. (the "Company", together with its subsidiaries, the "Group") is pleased to announce that the novel adjuvanted recombinant shingles vaccine REC610 independently developed by the Company has recently achieved positive...
Clover Announces Positive Phase Ⅰ Results for SCB-219M for Treatment of Chemotherapy-Induced Thrombocytopenia (CIT)
--All CIT patients maintained platelet counts >75 x 109/L at 1-week following chemotherapy plus a single dose of SCB-219M, with durable responses through at least 3-weeks-- --Durable efficacy and PK profile expected to support convenient dosing interval of ≥2-weeks, compared to daily or weekly d...
HANSIZHUANG Sets Sail in Indonesia Market
* The 1st China anti-PD-1 monoclonal antibody successfully approved in Southeast Asia - * The 1st overseas approval of HANSIZHUANG, highlighting another major milestone of Henlius' global strategy after HANQUYOU - * Along with KGbio and other partners to develop and launch HANSIZHUANG in ov...
RemeGen's Pioneering RC88 Receives IND Approval from US FDA for Platinum-Resistant Recurrent Ovarian Cancer Treatment
YANTAI, China, Dec. 28, 2023 /PRNewswire/ -- RemeGen Co. Ltd.
Innovent and Xuanzhu Enter into Clinical Trial Collaboration Investigating Combination Therapy of Sintilimab (PD-1 inhibitor) and A Novel ADC Candidate for Advanced Solid Tumors in China
ROCKVILLE, Md. and SUZHOU, China, Dec. 28, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology an...
Boan Biotech's Two Innovative Drugs Targeting Claudin 18.2 Granted ODD by FDA
YANTAI, China, Dec. 27, 2023 /PRNewswire/ -- Boan Biotech today announced that two of its Claudin18.2-targeted investigational drugs have been granted the Orphan Drug Designation (ODD) for the treatment of pancreatic cancer by the U.S. Food and Drug Administration (FDA): one is BA1105, an innovat...
Seegene obtains ISO45001 certification
SEOUL, South Korea, Dec. 27, 2023 /PRNewswire/ -- Seegene Inc. (KQ096530), a leading South Korean company providing a total solution for PCR molecular diagnostics, announced that it has achieved an ISO45001 certification on December 27. The Occupational Health and Safety Management System (ISO ...
Rx-360® Announces 2024 Board of Directors
PHILADELPHIA, Dec. 27, 2023 /PRNewswire/ -- Rx-360® is pleased to announce its 2024 Board of Directors. As a non-profit, the Board of Directors is positioned to continue driving the Rx-360® mission of pharmaceutical supply chain security, material quality, and patient safety. This announcement fo...
Innovent and SanegeneBio Enter Strategic Collaboration to Develop siRNA Drug for the Treatment of Hypertension
ROCKVILLE, Md. and SUZHOU, China, Dec. 27, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology an...
Davos Communications Awards - BGI Genomics Inclusive Instagram Strategy Wins Gold Recognition
HONG KONG, Dec. 22, 2023 /PRNewswire/ -- BGI Genomics, the world's leading provider of integrated solutions for precision medicine, is pleased to announce winning Instagram Campaign (Gold) recognition at the Davos Communications Awards held onDecember 12, 2023. Organized by the World Communicati...
International Clinical Trial Results Released! Responding to the Virus Resurgence, Lianhua Qingwen Capsules Provide New Evidence
SINGAPORE, Dec. 21, 2023 /PRNewswire/ -- Recently, according to multiple reports from local Singaporean media, confirmed cases of COVID-19 are steadily increasing, with the average number of daily hospitalizations on the rise. The virus, once vigorously fought against, seems to be making a comeba...
CStone sells to Servier its exclusive rights to TIBSOVO® in Greater China and Singapore
* The deal enables CStone to prioritize its resources to focus on the development of first-in-class and best-in-class therapies with global rights. * The deal enables Servier to potentially bring additional indications and accessibility of TIBSOVO® to patients in Greater China (including main...
Everest Medicines' Partner Calliditas Therapeutics Announces Nefecon® the Only FDA-approved Treatment for IgA Nephropathy to Significantly Slow Kidney Function Decline
SHANGHAI, Dec. 21, 2023 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company")'s licensing partner Calliditas Therapeutics AB (Nasdaq: CALT, Nasdaq Stockholm: CALTX) ("Calliditas") announced that the U.S. Food and Drug Administration (FDA) had approved Nefecon® delayed rele...
EpiVax Licenses ISPRI Toolkit to Eisai for Preclinical in silico Immunogenicity Screening
PROVIDENCE, R.I., Dec. 21, 2023 /PRNewswire/ -- EpiVax, Inc. ("EpiVax"), an internationally recognized leader in the field of immunogenicity, today announced that Eisai Co. Ltd. ("Eisai") has licensed EpiVax's ISPRI toolkit for preclinical immunogenicity risk assessment of Eisai's robust biologic...
Lynk Pharmaceuticals Announced First Rheumatoid Arthritis Patient Dosed in Phase Ⅲ Clinical Study of LNK01001
BOSTON and HANGZHOU and SHANGHAI, China, Dec. 20, 2023 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, announced that it has dosed the first patient with Rheumatoid Arthritis (RA) in a Phase Ⅲ clinical trial...
Burjeel Holdings Oncology Conference Marks 10 Years, Focusing on Equitable Solutions for Cancer Care
Experts stress collaboration and innovation are key to progress WASHINGTON, Dec. 19, 2023 /PRNewswire/ -- The 10th anniversary of the Burjeel Holdings Oncology Conference, held in partnership with Foreign Policy, brought together leading global experts to tackle the complex challenges of cancer ...
Week's Top Stories
Most Reposted
Agoda Launches Free Global eSIMs for VIP Diamond Members
[Picked up by 322 media titles]
2026-02-10 14:00DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 320 media titles]
2026-02-16 10:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Blackpanda Japan Announces Strategic Partnership with SoftBank to Strengthen Cyber Incident Response in Japan
[Picked up by 298 media titles]
2026-02-10 13:31Carro unveils quirky generative AI ad campaign highlighting its 'Surprisingly Short' AI-enabled car-selling process
[Picked up by 296 media titles]
2026-02-11 11:00