Pharmaceuticals
Antengene Enters into Commercialization Partnership with Hansoh Pharma for First/Only-in-Class XPO1 Inhibitor XPOVIO®(selinexor) in the Mainland of China
- Antengene and Hansoh Pharma to enter into collaboration agreement involving commercialization of XPOVIO® in the mainland of China, broadening coverage and improving access of the drug to patients in the mainland ofChina - Antengene to receive up to RMB200 million in upfront payments,...
First Patient Dosed in Pivotal Phase III Study of TLX591-CDx (Illuccix®) for Prostate Cancer Imaging in Chinese Patients
MELBOURNE, Australia, Aug. 11, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that a first patient has been dosed inChina in the pivotal Phase III registration study of TLX591-CDx (Illuccix®, Kit for the preparation of 68Ga-PSMA-11),[1] for the i...
YS Biopharma to Report First Quarter Fiscal Year 2024 Financial Results on August 15, 2023
GAITHERSBURG, Md., Aug. 10, 2023 /PRNewswire/ -- YS Biopharma Co., Ltd. (NASDAQ: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious disea...
AffaMed Therapeutics Announces Partner Vistagen Reports Positive Top-Line Results from Phase 3 PALISADE-2 Trial of Fasedienol (PH94B) Nasal Spray in Social Anxiety Disorder
First positive U.S. Phase 3 study of an investigational therapy for social anxiety disorder in over 15 years Statistically significant rapid-onset reduction in patient-reported Subjective Units of Distress Scale (SUDS) score compared to placebo in a public speaking challenge (primary endpoint, p...
Pfizer's Pneumococcal 20-Valent Conjugate Vaccine is Now Available in Hong Kong, For Adults Aged 18 Years or Older[1]
* First pneumococcal conjugate vaccine that fights against 20 serotypes leading to most of the invasive pneumococcal diseases and pneumonia[2],[3] — one of the leading causes of death inHong Kong[4] — has been approved by Hong Kong Department of Health inApril 2023 and is now available in Hong ...
Pharming announces first patient enrolled in Phase III clinical trial of leniolisib for the treatment of APDS in Japan
Single-arm Phase III study in Japan evaluating leniolisib in patients aged 12 years and older with APDS, a rare primary immunodeficiency LEIDEN, The Netherlands, Aug. 9, 2023 /PRNewswire/ -- Pharming Group N.V. ("Pharming" or "the Company") (EURONEXT Amsterdam: PHARM/Nasdaq: PHAR) announces that...
Everest Medicines to Announce First Half 2023 Financial Results on August 24, 2023
SHANGHAI, Aug. 9, 2023 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the development, manufacturing and commercialization of innovative medicines and vaccines, today announced that it will report financial results for the fir...
Lynk Pharmaceuticals Announced Positive Topline Data from Phase II Clinical Trial of LNK01001 in the Treatment of Atopic Dermatitis
HANGZHOU, China, Aug. 9, 2023 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, announced positive topline data from a Phase II clinical trial of LNK01001 for the treatment of atopic dermatitis. The study was...
Race Strategic Update August 2023
SYDNEY, Aug. 9, 2023 /PRNewswire/ -- Race Oncology Limited ("Race") is pleased to provide a strategic update, including an overview of revisions to corporate strategy, designed to optimise use of existing resources, while driving bisantrene's commercial partnering and collaboration potential. Ev...
Innovent Announces the Preclinical Results of IBI363 (PD-1/IL-2 Bispecific Antibody Fusion Protein) were Published in Nature Cancer
ROCKVILLIE, Md. and SUZHOU, China, Aug. 8, 2023 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and ...
Pfizer Malaysia, Sunway Medical Centre Velocity and Sunway Multicare Pharmacy Collaborate to Raise Awareness on the Importance of Early Detection of Atrial Fibrillation
KUALA LUMPUR, Malaysia, Aug. 8, 2023 /PRNewswire/ -- Pfizer Malaysia, Sunway Medical Centre Velocity and Sunway Multicare Pharmacy, have entered a tripartite collaboration on AF360, a program highlighting the importance of early detection of Atrial Fibrillation (AF). AF is a devastating heart dis...
First Patient Dosed in IPAX-2 Study of TLX101 Brain Cancer Therapy Candidate in Patients with Newly Diagnosed Glioblastoma
MELBOURNE, Australia, Aug. 8, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that a first patient has been dosed in a Phase I study of the Company's investigational therapy TLX101 (4-L-[131I] iodo-phenylalanine, or 131I-IPA) in combination with p...
Ligandal Inc. Appoints Tushar Nuwal as Chief Operating Officer and Chief Business Officer
NEW YORK, Aug. 7, 2023 /PRNewswire/ -- Ligandal Inc., an early-stage genetic medicine biotechnology company, announces the appointment of Tushar Nuwal as Chief Operating Officer and Chief Business Officer. Mr. Nuwal brings 20 years of biopharmaceutical experience, including roles at small and lar...
6-Month Registered Clinical Trial Data on World's First Transbronchial Pulmonary Radiofrequency Ablation System for Lung Cancer Announced, Showing Significant Efficacy
HANGZHOU, China, Aug. 7, 2023 /PRNewswire/ -- During the Chinese Medical Association 11th National Academic Conference on Respiratory Endoscopy and Interventional Pulmonology on August 5, Broncus (2216.HK) announced postoperative 6-month data of a registered clinical trial on RF II, the world's ...
Avance Clinical Earns Frost & Sullivan's 2023 Best Practices Customer Value Leadership Award for Delivering High-quality Clinical Trials Based on Globally Accepted Data
Avance Clinical is an Australian and North American market-leading Contract Research Organization (CRO) that provides international biotechs with customized solutions.For the fourth consecutive year, Avance Clinical has been awarded Best Practices Customer Value Leadership awarded, by Frost & Sul...
AI+ Media Development Platform AlfaOPA™ Officially Launched
HONG KONG, Aug. 4, 2023 /PRNewswire/ -- Today, the Future Technology: AI Empowering Bioprocessing Development summit hosted by Great Bay Bio (hereinafter referred to as "GBB") was successfully held at the Suzhou International Expo Center, where industry players gathered to celebrate the feast of...
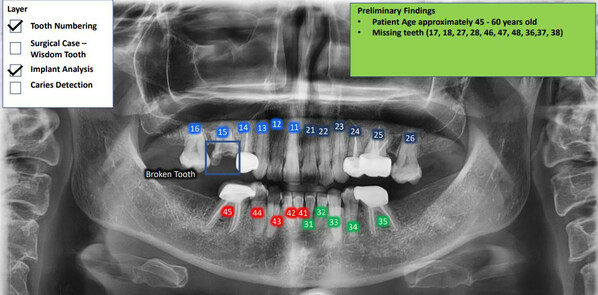
SayHeart Announces AI-Powered Visual-to-Text Transformation Pilot Testing for Dental X-Rays, with Kindlehouse Dentistry Partnership
KUALA LUMPUR, Malaysia, Aug. 3, 2023 /PRNewswire/ -- SayHeart, the groundbreaking health tech firm, is excited to announce the beginning of its pilot testing phase for a revolutionary visual-to-text transformation feature, focusing on dental X-ray images. This advanced AI-driven solution is aimed...
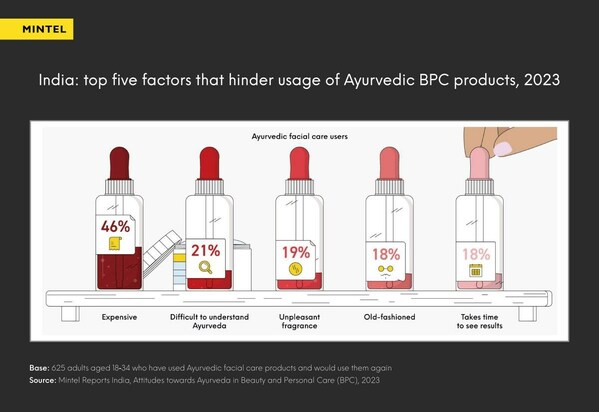
Interest in Ayurvedic beauty surges, but 1 in 5 Indian consumers find it old-fashioned
MUMBAI, India, Aug. 3, 2023 /PRNewswire/ -- Ayurveda, a centuries-old system of medicine originating inIndia, has witnessed a resurgence in popularity due to heightened consumer interest in self-care. However, the latest research from Mintel reveals that 1 in 5 Indian consumers still consider Ayu...
Unlocking the Power of Genetics: Camtech Unveils DNA Self-Test Kits for Holistic Wellness
SINGAPORE, Aug. 2, 2023 /PRNewswire/ -- Camtech Diagnostics, a provider of
innovative healthcare testing solutions, is excited to announce the launch of
its Ideal Health DNA Test
Selected for oral presentation! Kelun-Biotech to announce latest clinical study results of SKB264 (MK-2870, TROP2-ADC) for treatment of HR+/HER2- breast cancer at ESMO Congress 2023
CHENGDU, China, Aug. 1, 2023 /PRNewswire/ -- Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. ("Kelun-Biotech", HKEX: 6990.HK) announced that it will present the data from a Phase I/II clinical trial of the innovative TROP2-ADC (SKB264, MK-2870) jointly developed by Kelun-Biotech and MSD (the t...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 317 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00
![Pfizer's Pneumococcal 20-Valent Conjugate Vaccine is Now Available in Hong Kong, For Adults Aged 18 Years or Older[1]](https://mma.prnasia.com/media2/2155046/Pfizer.jpg?p=medium600)