Biotechnology
WuXi ATU Opens New Manufacturing Facility to Further Enhance Its CTDMO Services for Global Customers
PHILADELPHIA, Oct. 17, 2021 /PRNewswire/ -- WuXi Advanced Therapies Inc. (WuXi ATU) – a wholly owned subsidiary of WuXi AppTec – today announced the opening of its new process development and commercial manufacturing facility in Lin-gang, Shanghai. WuXi ATU is a leading Contract, Testing, Develop...
CellOrigin secured a new round of investment for developing its globally proprietary iPSC-CAR-Macrophage technology platform
HANGZHOU, China, Oct. 15, 2021 /PRNewswire/ -- On Oct. 11th, 2021, CellOrigin Inc. released data about its second generation of iPSC-CAR-Macrophage which has a genetically integrated secondary signal to confer controlled CAR-iMac polarization, in the 5th International Conference of IGC China, 202...
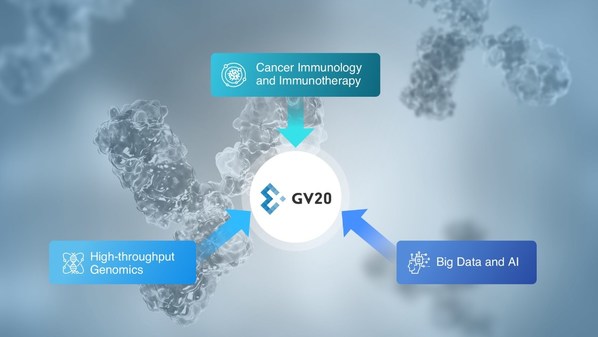
GV20 Oncotherapy Completes Series B Financing to Advance Pipeline into the Clinic and Expand Immuno-Oncology Drug Discovery Platform
SHANGHAI, Oct. 14, 2021 /PRNewswire/ -- GV20 Oncotherapy, a biotechnology company with unique expertise in novel target identification and antibody drug discovery in immuno-oncology, today announced the completion of its Series B financing. This round of financing was led by Coatue Management,...
BioAgilytix Closes Acquisition of Australia-based 360biolabs®
Following final government approval, 360biolabs, a BioAgilytix company, adds world-class virology & immunology expertise and LC/MS/MS small molecule capabilities to the company, enabling support of bioanalytical services across all geographies and development stages DURHAM, N.C., Oct. 14, 2021 ...
I-Mab Advances Late-stage Development of Its Differentiated CD38 Antibody Felzartamab (TJ202) in China
* Completed patient enrollment in phase 3 registrational trial of felzartamab in combination with lenalidomide for second-line treatment of multiple myeloma (MM) * Felzartamab for MM third-line treatment is on track for BLA submission in Q4 2021 * New IND application for combination of felz...
AUM Biosciences announces closing of USD $27Million in a "Series A" Round of funding to advance its clinical stage pipeline of precision and targeted cancer therapies
SINGAPORE, Oct. 12, 2021 /PRNewswire/ -- AUM Biosciences (AUM) today announced the successful completion of USD$27 million series A funding round. This will fuel AUM's vision of developing a world class biotech pipeline focused on drugging what many consider as the undruggable targets, as well ...
NeoPlex (TM) HPV29 Detection, a new molecular detection kit from Genematrix, acquires European CE-IVD certification
* Real-time PCR-based human papillomavirus (HPV) detection product is launched. * Simultaneous HPV detection and genotyping of 29 HPV types are available. PANGYO,South Korea, Oct. 12, 2021 /PRNewswire/ -- Genematrix (KRX 109820), specialized in real-time PCR based molecular diagnostics, annou...
ProfoundBio Announces Presentation on Novel, Proprietary Linker-Drug Technology to Enable Antibody-Drug Conjugates (ADCs) with an Expanded Therapeutic Window at the AACR-NCI-EORTC International Conference
WOODINVILLE, Wash. and SUZHOU, China, Oct. 7, 2021 /PRNewswire/ -- ProfoundBio announces that preclinical data from its novel, proprietary ADC technology platform are being presented at the 2021 Molecular Targets and Cancer Therapeutics conference hosted by the American Association for Cancer Res...
Amplo Biotechnology Closes Series Seed Financing to Advance Gene Therapies for The Treatment of Neuromuscular Junction Disorders
NEW YORK, Oct. 6, 2021 /PRNewswire/ -- Amplo Biotechnology, a privately held US-based biotech focused on developing AAV (Adeno-associated Virus)-based gene therapies for diseases affecting the neuromuscular junction, today announced the successful closing of an undisclosed Series Seed financing. ...
I-Mab Announces Upcoming Participation at October Conferences
SHANGHAI and GAITHERSBURG, Md., Oct. 1, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced its participation in the following conferences in October...
Binhai sets ambitious biomedical goals with support of key companies
TIANJIN, China, Sept. 30, 2021 /PRNewswire/ -- A news report by chinadaily.com.cn: The Binhai New Area in North China's port city of Tianjin has gathered together more than 1,500 biomedical companies that have made great scientific achievements in key areas, such as recombinant proteins and stem...
Penn mRNA Biology Pioneer Drew Weissman Joins RVAC's Science Advisory Board
SINGAPORE, Sept. 30, 2021 /PRNewswire/ -- RVAC Medicines Pte. Ltd. ("RVAC"), an emerging messager RNA (mRNA) platform company inSingapore aspiring to address various diseases with unmet medical needs for emerging markets through novel vaccines and therapeutics, today announced that one of the to...
I-Mab Reports Multiple Positive Clinical Updates of Differentiated CD47 Antibody Lemzoparlimab
* The preliminary efficacy and safety data from the phase 2 U.S. trial in NHL has been submitted for presentation at ASH 2021The current U.S. NHL clinical trial has now expanded to include clinical sites inChina as an international multi-center clinical trial, which will potentially lead to a r...
GenFleet and Innovent Starts First-in-human Phase I/II Trial of KRAS G12C Inhibitor
GUANGZHOU, China, Sept. 29, 2021 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, and Innovent Biologics (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercialize...
Neurophth Announces the Completion of GMP Manufacturing Facility for Gene Therapy Products According to International Standards
WUHAN, China and SAN DIEGO, Sept. 27, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd., a fully-integrated genomic medicines company seeking to improve lives through the curative potential of gene therapies, announced the opening of its state-of-the-art manufacturing facility at the Phase II Suz...
Harbour BioMed Announces Dosing of First Patient of Batoclimab Phase III Trial in Patients with Generalized Myasthenia Gravis
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, Sept. 27, 2021 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) announced that, the clinical trial of its anti-FcRn antibody, batoclimab (HBM9161), has completed the first dosing of first patient in registrational phase III trial f...
Senhwa Announces Acceptance of Early Positive COVID-19 Phase II Study Abstract for Presentation at the ISIRV-WHO Conference
* Early Positive Phase 2 efficacy and safety data are both statistically significant and clinically meaningful. * No SAE related to Silmitasertib was reported. * Silmitasertib is a host-directed antiviral and an anti-inflammatory investigational therapy expected to be effective against eme...
Transcenta Received IND Clearance from NMPA of its Anti-sclerostin Monoclonal Antibody TST002
SUZHOU, China, Sept. 26, 2021 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta"), a clinical stage biopharmaceutical company with fully-integrated capabilities in discovery, research, development and manufacturing of antibody-based therapeutics, announces that it received IND clearance on...
GenScript ProBio and AskGene Enter into a Non-exclusive License of A sdAb Targeting Immune Checkpoint Target
NANJING, China, Sept. 26, 2021 /PRNewswire/ -- On September 26, 2021, GenScript ProBio and AskGene signed a license agreement forGenScript ProBio's single-domain antibody (sdAb) targeting an immune checkpoint target.GenScript ProBio grants a non-exclusive global license to AskGene to use GenScrip...
Boan Biotech to Present the Pre-clinical Data of Its Proprietary CD3+ T-cell Engager Platform and CEA/CD3 Bispecific Antibody at World Bispecific Summit
Sept. 25, 2021, BOSTON /PRNewswire/ -- Boan Biotech, a subsidiary of Luye Pharma Group, will orally present the pre-clinical data of its proprietary CD3+ T-cell engager platform and CEA/CD3 bispecific antibody at World Bispecific Summit 2021. The annual World Bispecific Summit aiming to promote ...
Week's Top Stories
Most Reposted
Supported by the Luxembourg Government, The Luxembourg House of Financial Technology (LHoFT) and the Asian Development Bank (ADB) Announce the 2nd Edition of Catapult | SE Asia 2025
[Picked up by 313 media titles]
2025-01-22 15:38Blackpanda Recognized with Frost & Sullivan's Asia Pacific Company of the Year Award for Incident Response Excellence
[Picked up by 302 media titles]
2025-01-23 09:00Agoda Expands Its Eco Deals Program Pledging Up to $1.5 Million to Fund Critical Conservation Projects Across 10 Asian Markets in Partnership with WWF
[Picked up by 292 media titles]
2025-01-23 09:00Sephora Unveils Its First Film, "Beauty & Belonging" Celebrating Diversity and Authenticity
[Picked up by 288 media titles]
2025-01-24 08:00Star-Studded Sponsor Line-up Takes Centre Court at the Singapore Tennis Open 2025
[Picked up by 287 media titles]
2025-01-28 10:49