Biotechnology
Henlius reports half-year (H1) 2021 results, outlines remarkable growth in commercialization and needs-driven innovation
SHANGHAI, Aug. 18, 2021 /PRNewswire/ -- Henlius (2696.HK) announced Half-Year (H1) results endedJune 30th, 2021, sharing the company's recent noteworthy progress and achievements. As a global innovative biopharmaceutical company, Henlius is committed to offering high-quality, affordable and innov...
PharmAbcine to Participate in Biotechgate Digital Partnering
DAEJEON, South Korea, Aug. 17, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next generation antibody therapeutics, announced today that the Company will participate in Biotechgate Digital Partnering taking place virtuall...
First Edition of World Conference on Early Detection of Cancer Set to Start in November in Hainan, China
GUANGZHOU and HAIKOU, China, Aug. 16, 2021 /PRNewswire/ -- Early detection and diagnosis of cancer is an essential enabler of curative treatment and an effective solution to boost the survival rates of cancer patients worldwide. In an effort to lead the way in innovative research and strategies f...
Gracell Biotechnologies Signs Exclusive License Agreement with FutureGen Biopharm to Develop Engineered Immune Cell Therapies Targeting Claudin 18.2 in Solid Tumors
SUZHOU and SHANGHAI, China, and PALO ALTO, Calif., Aug. 16, 2021 /PRNewswire/ -- Gracell Biotechnologies Inc. (NASDAQ: GRCL) ("Gracell"), a global clinical-stage biopharmaceutical company dedicated to developing highly efficacious and affordable cell therapies for the treatment of cancer, today ...
Senhwa Completes Enrollment of a Phase 2 Investigator Initiated Trial of Silmitasertib as Novel Oral Drug for COVID-19
TAIPEI and SAN DIEGO, Aug. 16, 2021 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a new drug development company focused on human efficacy and innovation of first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, today announced that its novel oral drug, Silmita...
GeneQuantum announces a key leadership appointment
SUZHOU, China, Aug. 15, 2021 /PRNewswire/ -- GeneQuantum Healthcare, an industry leading biopharmacetucial company dedicated to next generation bioconjugate drugs through innovative intelligent ligase-dependent conjugation (iLDC) technology platform, announced a key appointment to further strengt...
InxMed's IN10018 Receives U.S. FDA Fast Track Designation for the Treatment of Platinum-resistant Ovarian Cancer
NANJING, China, Aug. 15, 2021 /PRNewswire/ -- InxMed (Nanjing) Co., Ltd. ("InxMed" or "Company"), a clinical stage biotech company dedicated to developing innovative, individualized medicines with international impact, announced today the U.S. Food and Drug Administration (FDA) has granted Fas...
Arctic Vision Announces Expansion of License Territory for Suprachoroidal Space Injection Therapy ARVN001 to Include ASEAN Countries and India
SHANGHAI, Aug. 15, 2021 /PRNewswire/ -- Arctic Vision, a China-based biotech company focused on innovative ophthalmic therapies, announced today the entering of an amendment to the exclusive license agreement with its partner Clearside Biomedical, Inc. (Nasdaq: CLSD) ("Clearside"), to expand the ...
WuXi AppTec Reports Strong 2021 Interim Results
* Revenue Up 45.7% Year-Over-Year to RMB10,537 Million * Net Profit Attributable to Owners of the Company Up 55.8% Year-Over-Year to RMB2,675 Million * Diluted EPS Up 46.8% Year-Over-Year to RMB0.91 * Adjusted Non-IFRS[1] Net Profit Attributable to Owners of the Company Up 67.8% Year-Over-...
NUHS Embarks on Holomedicine Research in Singapore, Using Mixed Reality Technology to Enhance Diagnosis, Education and Patient Care
SINGAPORE, Aug. 12, 2021 /PRNewswire/ -- To be able to literally "see inside" a
patient's skull and identify the location of the tumour deep in the brain,
right on the operating table, may no longer be science fiction with the help of
a Mixed Reality (MR) headset.
ImmVira's MVR-T3011 IV Completed First Dosing for Intravenous Administration in a U.S. Phase I Clinical Trial
SHENZHEN, China, Aug. 10, 2021 /PRNewswire/ -- ImmVira announced that it has initiated its lead oncolytic virus therapy program MVR-T3011 IV (also known as T3011). The first patient has been dosed, receiving MVR-T3011 intravenous (IV) administration in the U.S. onAugust 10, 2021. The Phase I clin...
Belief Biomed's Gene Therapy for Hemophilia B Receives NMPA IND Approval: First Intravenous Infusion Gene Therapy for Rare Disease in China
SHANGHAI, Aug. 10, 2021 /PRNewswire/ -- On August 10th, 2021,Belief Biomed announced thatChina's National Medical Products Administration (NMPA) has cleared the IND (Investigational New Drug) application for Belief Biomed's BBM-H901. This marks the first IND approval for Intravenous (i.v.) infusi...
INDICAID(TM) COVID-19 Rapid Antigen Test Receives Emergency Use Authorization from the U.S. Food and Drug Administration
GARDEN GROVE, Calif., Aug. 10, 2021 /PRNewswire/ -- PHASE Scientific
International LTD
FibroBiologics Announces Inaugural Board of Directors
Company Announces Formation of Board of Directors HOUSTON, Aug. 6, 2021 /PRNewswire/ -- FibroBiologics, a clinical stage company developing fibroblast-based therapeutic solutions for chronic diseases, announced today the formation of its Board of Directors. Leading this group is Chairman,Pete ...
ABL Bio Receives IND Approval for Phase 1 Clinical Trial of ABL501, an anti-LAG-3/PD-L1 Bispecific Antibody
* South Korea's MFDS clears IND application for a Phase 1 study evaluating the safety and preliminary efficacy of ABL501 as a monotherapy for solid tumor treatment * ABL's third IND this year for bispecific antibody therapeutics SEONGNAM, South Korea, Aug. 4, 2021 /PRNewswire/ -- ABL Bio, Inc...
Alterity Therapeutics Announces New US Patent to Expand its Portfolio of Compounds for Neurodegenerative Diseases including Alzheimer's and Parkinson's
MELBOURNE, Australia, Aug. 4, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative conditions, today announced the United States Patent and Trademark Of...
Happiness Biotech Announced Financial Results for Fiscal Year Ended March 31, 2021
NANPING, China, Aug. 2, 2021 /PRNewswire/ -- Happiness Biotech Group Limited (the "Company" or Nasdaq: HAPP), an innovativeChina-based nutraceutical and dietary supplements producer and e-commerce services provider, today announced its financial results for the fiscal year endedMarch 31, 2021. F...
Bionomics Announces Plans to Conduct U.S. Initial Public Offering
ADELAIDE, Australia, Aug. 2, 2021 /PRNewswire/ -- Bionomics Limited (ASX: BNO, OTCQB: BNOEF), (Bionomics) a global, clinical stage biopharmaceutical company, is pleased to announce that it plans to conduct a registered initial public offering of American Depositary Shares (ADSs) in the United ...
BIOKANGTAI COVID-19 Vaccine Approved for Phase III Clinical Trial in the Philippines
SHENZHEN, China, Aug. 2, 2021 /PRNewswire/ -- On July 30, Shenzhen Kangtai Biological Products Co., Ltd. (BIOKANGTAI) was approved by the Philippine Food and Drug Administration (PFDA) to launch the phase III clinical trial of its independently developed Inactivated COVID-19 Vaccine (Vero cells) ...
LISCure Biosciences Announces Know-How License Agreement and Stock Purchase Agreement with Mayo Clinic
SEOUL, South Korea, Aug. 1, 2021 /PRNewswire/ -- LISCure Biosciences, Inc. ("LISCure") has entered into a know-how license and development collaboration agreement, and stock purchase agreement with Mayo Clinic to advance LISCure's microbiome therapeutics in non-alcoholic steatohepatitis (NASH). ...
Week's Top Stories
Most Reposted
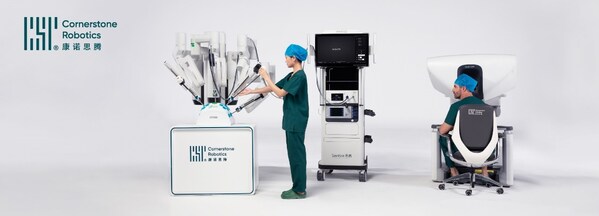
Cornerstone Robotics Raises over US$70 million Funding to Forge Accessibility in Robotic Surgery
[Picked up by 323 media titles]
2025-01-13 09:05URBAN REVIVO Celebrates Lunar New Year with Unique Collaboration with Artist Mercedes Bellido
[Picked up by 294 media titles]
2025-01-13 10:44RuggON Launches VIKING II: Transforming Fleet Management with Unmatched Technology and Flexibility
[Picked up by 287 media titles]
2025-01-14 21:00Gen Z Travelers Drive Buy Now, Plan Later Travel Bookings by Over 20%: Fliggy's 2024 Buy Now Plan Later Travel Insight
[Picked up by 286 media titles]
2025-01-10 16:04VANTAGE FOUNDATION SUPPORTS GRAB INDONESIA IN EMPOWERING WOMEN DRIVER-PARTNER
[Picked up by 280 media titles]
2025-01-09 18:26