Biotechnology
Senhwa's Silmitasertib, COVID-19 Drug Candidate, Receives Positive Interim Review from Data Monitoring Committee to Proceed
TAIPEI and SAN DIEGO, Aug. 31, 2021 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focused on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, today announced that its oral new drug, Silmitasertib, had received a positive feedbac...
I-Mab Provides Business and Corporate Updates and Reports Financial Results for the Six Months Ended June 30, 2021
* 13 significant clinical milestones achieved year-to-date, including critical positive data readout events for felzartamab (TJ202/MOR202), lemzoparlimab (TJC4), uliledlimab (TJD5), and plonmarlimab (TJM2) and more milestone achievements are expected before the year-end 2021 * Felzartamab (TJ...
Harbour BioMed Reports 2021 Interim Results
CAMBRIDGE, Mass.and SUZHOU, China and ROTTERDAM, Netherlands, Aug. 31, 2021 /PRNewswire/ -- Harbour BioMed ("HBM", or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics, reported its interim...
Through CEO of Hanmi Science, CancerRop Issues KRW 20 Billion New Shares for Cooperation with Oxford Vacmedix
SEOUL, South Korea, Aug. 30, 2021 /PRNewswire/ -- CancerRop (180400), a company specializing in precision medicine and molecular diagnostics, announced a jump start in the global vaccine hub for COVID-19 mRNA (messenger ribonucleic acid) with Hanmi Science through20 billion won worth of investmen...
Deebio's Global Bio-enzyme Journey: 27 Years, 30 Countries and Regions
DEYANG, China, Aug. 30, 2021 /PRNewswire/ -- Sichuan Deebiotech Co., Ltd. (hereinafter referred to as Deebio) recently celebrated its 20th anniversary of uninterrupted exports of their products to the EU during the 86th API China. The company's European partners sent congratulatoryvideo, while ...
GeneQuantum announces Dr. Yi Xia as Senior Vice President of Statistics and Data Science
SUZHOU, China, Aug. 29, 2021 /PRNewswire/ -- GeneQuantum Healthcare, an industry leading biopharmaceutical company dedicated to next generation bioconjugate drugs through innovative intelligent ligase-dependent conjugation (iLDC) technology platform, announces the appointment of Dr.Yi Xia as Seni...
Triad Life Sciences completes A$25m funding round, anticipates ASX listing in coming months
MEMPHIS, Tenn., Aug. 29, 2021 /PRNewswire/ -- Innovative US based biotech companyTriad Life Sciences, Inc (Triad or the Company), is pleased to announce the completion of a successfulA$25 million funding round which saw strong support from major Australian institutional investors. The convertible...
InnoCare Releases 2021 Half Year Results and Business Highlights
BEIJING, Aug. 29, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a leading biopharmaceutical company focusing on cancer and autoimmune diseases, today announced 2021 half year results which ended onJune 30, 2021. Dr. Jasmine Cui, Co-founder, Chairwoman and CEO of InnoCare, said, "On the fo...
3SBio announces 2021 interim results, with strong growth in core businesses and significant progress in clinical development
HONG KONG, Aug. 26, 2021 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today unveiled its 2021 interim results. 3SBio maintained strong performance in core businesses, with core products occupying a leading market share. Meanwhile, the Company continuously increased ...
Tigermed Reports 2021 Interim Results with Strong Financial Growth
HANGZHOU, China, Aug. 25, 2021 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. (stock code: 300347.SZ / 3347.HK), a leading provider of innovative clinical research solutions for biopharmaceutical and medical device industry, reports its interim results for the six months endedJune 30, 202...
ExoCoBio to Build the World's First Clinical Grade Exosome GMP Manufacturing Facility
* $20 million investment for a new Mfg & R&D facility * Strategic platform for partnering & CDMO business SEOUL, South Korea, Aug. 25, 2021 /PRNewswire/ -- ExoCoBio Inc., the 4th largest exosome player in the world, announced the initiation of its "Project EGMP", starting with the constructio...
GenScript Biotech Reports First Half 2021 Results
* In the first half of 2021, the Group reported a revenue of approximately USD 229.6 million, a 38.0% YoY increase, and a gross profit of USD 138.6 million , achieving a 28.1% YoY growth. All four of the Group's business segments demonstrated steady growth. * Building on early strategic invest...
Kintor Pharma to Report 2021 Interim Financial Results and Host Conference Call on August 30, 2021
SUZHOU, China, Aug. 24, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, announced today the company will review the interim results and business highlights on...
Hepagene Therapeutics, Inc. Announces Positive Results from Phase I Trial of HPG1860
SHANGHAI, Aug. 23, 2021 /PRNewswire/ -- Hepagene Therapeutics, Inc., a clinical stage biopharmaceutical company focusing on novel therapies for patients with liver diseases, today announced positive results from its Phase I study of HPG1860 conducted inthe United States. HPG1860 is a non-bile aci...
PharmAbcine to Participate in H.C. Wainright 23rd Annual Global Investment Conference
DAEJEON, South Korea, Aug. 23, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next generation antibody therapeutics, announced today that the Company will participate in H.C. Wainright 23rd Annual Global Investment Confere...
Roche and KeChow Reach a Cooperation Agreement
SHANGHAI, Aug. 20, 2021 /PRNewswire/ -- On August 2, 2021, Shanghai Roche Pharmaceuticals Co., Ltd. (hereinafter referred to as "Roche Pharma China" or "Roche") and Shanghai KeChow Pharma, Inc. (hereinafter referred to as "KeChow Pharma" or "KeChow") entered into a cooperation agreement to improv...
Neurophth Expands Its Global Gene Therapy Leadership Team with the Appointment of Dr. Zhengbin Li as Head of Commercial
WUHAN, China and SAN DIEGO, Aug. 19, 2021 /PRNewswire/ -- Neurophth Biotechnology Ltd., a fully-integrated genomic medicines company committed to the development of AAV-delivered gene therapies for the treatment of ocular diseases, today announced the recent appointment of Dr. Zhengbin (Luke) Li ...
Daewoong Pharmaceutical and Hanall Biopharma Invest $1M USD in Alloplex Biotherapeutics
SEOUL, South Korea, Aug. 19, 2021 /PRNewswire/ -- Daewoong Pharmaceutical and
Hanall Biopharma ofSouth Korea are expanding their global open collaboration
initiative by investing in Alloplex Biotherapeutics, an emergingBoston-based
biotechnology company.
PharmAbcine Announces Submission of PCT for an Anti-ANG2 Antibody for the Treatment of Ocular Diseases
DAEJEON, South Korea, Aug. 19, 2021 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next-generation antibody therapeutics, today announced it submitted a Patent Cooperation Treaty (PCT), an international patent application, for ...
MicroConstants to Join BioAgilytix Family
Highly complementary transaction strengthens BioAgilytix's ability to support bioanalytical services across all major therapeutics in development; adds LC/MS/MS capabilities; and expands team of seasoned scientists DURHAM, N.C., Aug. 18, 2021 /PRNewswire/ -- BioAgilytix Labs, LLC (BioAgilytix),...
Week's Top Stories
Most Reposted
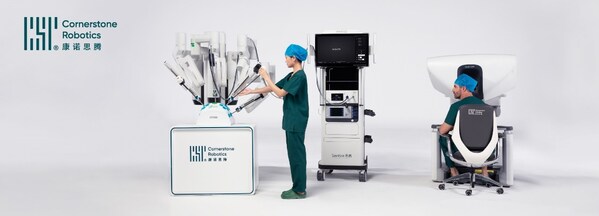
Cornerstone Robotics Raises over US$70 million Funding to Forge Accessibility in Robotic Surgery
[Picked up by 323 media titles]
2025-01-13 09:05URBAN REVIVO Celebrates Lunar New Year with Unique Collaboration with Artist Mercedes Bellido
[Picked up by 294 media titles]
2025-01-13 10:44RuggON Launches VIKING II: Transforming Fleet Management with Unmatched Technology and Flexibility
[Picked up by 287 media titles]
2025-01-14 21:00Gen Z Travelers Drive Buy Now, Plan Later Travel Bookings by Over 20%: Fliggy's 2024 Buy Now Plan Later Travel Insight
[Picked up by 286 media titles]
2025-01-10 16:04VANTAGE FOUNDATION SUPPORTS GRAB INDONESIA IN EMPOWERING WOMEN DRIVER-PARTNER
[Picked up by 280 media titles]
2025-01-09 18:26