Medical/Pharmaceuticals
Breakthrough in Long COVID: An investigator-initiated trial (IIT) with Hyundai Bioscience's Xafty by UCSD
SAN DIEGO, Sept. 17, 2024 /PRNewswire/ -- Hyundai Bioscience USA announced that it has signed an MOU with theUniversity of California San Diego (UCSD) to conduct an investigator-initiated trial to evaluate the efficacy of Xafty®, a niclosamide-based antiviral developed by its Korean headquarters,...
Datasea's New 5G AI Multimodal Agreements Have Already Generated $9.0 Million of 5G-AI Services
Agreements Boost Fiscal 2025 Revenue and Expand Datasea's 5G Application Market Positioning inChina BEIJING, Sept. 17, 2024 /PRNewswire/ -- Datasea Inc. (NASDAQ: DTSS) ("Datasea" or the "Company"), aNevada-based company focused on innovative high-tech acoustics and 5G-Artificial Intelligence ("A...
See-Mode Technologies Receives FDA Clearance for Thyroid Ultrasound AI Analysis and Reporting Software
MELBOURNE, Australia, Sept. 17, 2024 /PRNewswire/ -- See-Mode Technologies, a global innovator in AI for ultrasound imaging, today announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) for their thyroid ultrasound analysis and reporting software. See-Mode's A...
Subgroup Analysis from Pivotal WU-KONG1B Study Exhibits Robust Efficacy of Sunvozertinib in Non-Small Cell Lung Cancer Patients with EGFR Exon 20 Insertion Mutations Across Different Baseline Characteristics
Results of subgroup analysis from the pivotal WU-KONG1B study in relapsed or refractory NSCLC with EGFR exon20ins presented at ESMO 2024 * Sunvozertinib demonstrated promising anti-tumor efficacy, regardless of EGFR exon20ins region classification, race, region, baseline brain metastasis, prio...
WestGene Biopharma Presents Groundbreaking mRNA Vaccine Data at ESMO 2024
BARCELONA, Spain, Sept. 16, 2024 /PRNewswire/ -- WestGene Biopharma, a leading innovator in mRNA therapeutics, presented the latest clinical data for its EBV-positive tumour mRNA vaccine, WGc-043, during a mini-oral presentation at the European Society for Medical Oncology (ESMO) Congress 2024. ...
Concord Medical Announces Obtaining Large Medical Equipment Procurement License for Its Proton Therapy Equipment
BEIJING, Sept. 16, 2024 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specialized in cancer treatment, research, education and prevention inChina, today announced that Guangzhou Concord Cancer Center ("Guangzhou H...
Vazyme Leads Life Science Innovation, Boosting Research Breakthroughs in mIDH1 Cancer Treatment
NANJING, China, Sept. 16, 2024 /PRNewswire/ -- Vazyme (688105.SH), a leading life science technology company, significantly contributed to groundbreaking research published in Science Magazine through its innovative products. The study, which focuses on how mutant IDH1 inhibition activates tumor ...
Antennova Releases Latest Data of CD73 Inhibitor ATN-037, including a DCR of 89.5%, in a Mini Oral at ESMO Congress 2024
* In patients with non-small cell lung cancer (NSCLC) or melanoma who had acquired resistance to checkpoint inhibitors (CPIs), ATN-037 in combination with KEYTRUDA®(pembrolizumab) demonstrated an overall response rate (ORR) of 21.1% and a disease control rate (DCR) of 89.5%. * Data from the...
Akeso's Ivonescimab plus Chemo in First-Line Triple-negative Breast Cancer Showed Promising Preliminary Efficacy and Good Safety at ESMO 2024
HONG KONG, Sept. 16, 2024 /PRNewswire/ -- At the 2024 European Society for Medical Oncology (ESMO) Conference, Akeso (9926.HK) published the phase 2 results from its ivonescimab in combination with chemotherapy as a first-line (1L) treatment for triple-negative breast cancer (TNBC). The prelimina...
Akeso Published Ivonescimab plus Ligufalimab as First-Line Treatment for PD-L1-Positive Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma at ESMO 2024
Head-to-Head Phase 3 Trial Compared to Pembrolizumab Initiated HONG KONG, Sept. 15, 2024 /PRNewswire/ -- At the 2024 European Society for Medical Oncology (ESMO) Conference, Akeso released the Phase 2 clinical results of its internally developed PD-1/VEGF bispecific antibody, ivonescimab, with or...
Dialogue with CGT Leaders! You Are Invited to Join the "2024 GenScript Biotech Global Forum•London" to Discuss the Future Together
PISCATAWAY, N.J., Sept. 14, 2024 /PRNewswire/ -- The year 2024 marks a milestone year for continuous advancements in Cell and Gene Therapy (CGT). The global launch of Amtagvi, a Tumor-Infiltrating Lymphocyte (TIL) therapy, has ushered in a new era for solid tumor treatment using cell therapy. PM3...
Breakthrough Therapy designation for Sanbexin sublingual tablets granted by the United States Food and Drug Administration
NANJING, China, Sept. 14, 2024 /PRNewswire/ -- On September 2, 2024, Simcere Pharmaceuticals Group Ltd. (2096.HK) announced that Sanbexin Sublingual Tablets (Edaravone and Dexborneol sublingual tablets), an innovative drug for stroke, has been granted the Breakthrough Therapy designation by the U...
Harbour BioMed Announces the Latest Clinical Data on the First-in-Class Fully Human Anti-B7H7/HHLA2 Monoclonal Antibody HBM1020 at the ESMO Congress 2024
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Sept. 14, 2024 /PRNewswire/ -- Harbour BioMed (the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology and immuno...
MediLink presents YL201 (B7H3 ADC) at ESMO 2024, with over 6-months PFS in SCLC, and showing pan-tumor benefits
* The first publication of clinical data for YL201, featured in an oral presentation at ESMO 2024. * Encouraging antitumor activity of YL201 in multiple solid tumor types, including SCLC, NPC, and wild-type NSCLC, from Phase I escalation and expansion results. * In extensive-stage SCLC pati...
111 Inc. Announces Its Co-founders' Strategic Share Purchase and Highlights Continued Growth and Innovation
SHANGHAI, Sept. 13, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to reshaping the value chain of healthcare industry by digitally empowering the upstream and downstream inChina, today was informed by co-founders...
US FDA Grants RPD Designation to Senhwa Biosciences Silmitasertib for Pediatric Neuroblastoma
TAIPEI and SAN DIEGO, Sept. 13, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a new drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced that its new drug Silmitasertib (CX-4945) was granted a rare pe...
Nona Biosciences Enters into Collaboration Agreement with Umoja Biopharma to Advance In Vivo CAR-T Cell Therapies
CAMBRIDGE, Mass., Sept. 12, 2024 /PRNewswire/ -- Nona Biosciences, a global biotechnology company providing a total solution from "Idea to IND" (I to ITM), announced today that it has entered into a multi-target antibody discovery collaboration with Umoja Biopharma, a transformative immunotherapy...
Stapokibart Was Granted Marketing Approval From National Medical Products Administration For The Treatment Of Moderate-to-severe Atopic Dermatitis in Adults
CHENGDU, China, Sept.12, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced the National Medical Products Administration (the "NMPA") ofChina has recently approved the new drug application for Stapokibart (anti-IL-4Rα monoclonal antibody, trade name:Kangyueda (康悦达), for th...
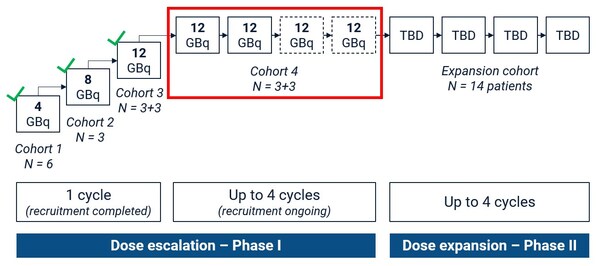
SECuRE trial advances: No dose limiting toxicities and strong preliminary efficacy data in first multi-dose cohort
Highlights * Cohort 4 of the SECuRE trial is the first to assess multiple cycles of 67 Cu-SAR-bisPSMA at the highest dose of 12GBq. * The Safety Review Committee (SRC) assessed early data from the first 3 participants in cohort 4 who received 2 doses of67Cu-SAR-bisPSMA. Two of these participa...
E-Home Household Services Holdings Limited's subsidiary Zhongrun Pharmaceutical and New Zealand's NBL Pharmaceuticals Sign Cooperation Agreement to Expand International Markets
FUZHOU, China, Sept. 12, 2024 /PRNewswire/ -- E-Home Household Services Holdings Limited (NASDAQ:EJH) (the "Company" or "eHome"), an integrated home services provider inChina, announced today that after the detailed business and market due diligence conducted by the Company and the detailed busin...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 317 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00