Medical/Pharmaceuticals
GenScript Appoints New Board Members to Drive Global Growth
PISCATAWAY, N.J., Sept. 12, 2024 /PRNewswire/ -- GenScript Biotech Corporation ("GenScript"), a global leader in life sciences products and services, announced the appointment of Dr.Ross Allen Grossman as a Non-Executive Director and Dr.Alphonse Galdes as an Independent Non-Executive Director. Th...
Bloomage 2024 Mid-Year Report: International Raw Materials Sales Surpass Domestic Chinese Market's for the First Time
PARSIPPANY, N.J., Sept. 11, 2024 /PRNewswire/ -- Bloomage, a global leader in hyaluronic acid and other bioactive substance innovations, showcased robust growth in its raw materials business in its newly released 2024 mid-year financial report. The company reported a total revenue ofRMB 2.811 bil...
Collagen tripeptides: a new era of collagen drinks
Collagen is highly valued not only for its anti-aging properties but also for its potential benefits in promoting joint health, weight management, and improving overall wellbeing. TAIPEI, Sept. 11, 2024 /PRNewswire/ -- The 2023 global collagen market was valued atUSD 9.76 billion and is projecte...
TCI Revolutionizes Weight Management with Breakthrough GLP-1 Innovation
TAIPEI, Sept. 11, 2024 /PRNewswire/ -- TCI Co. Ltd. ("TCI", 8436.TWO), a global leader in ODM health and cosmetic products for over 40 years, introduces its cutting-edge GLP-1 formula, a natural blend of plant extracts, probiotics, and prebiotics designed to deliver powerful weight management res...
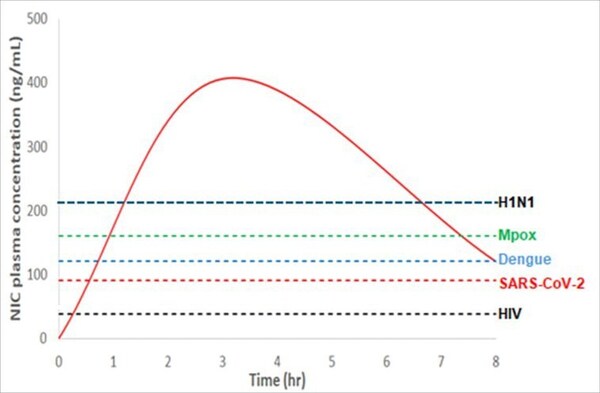
Hyundai Bioscience announces XAFTY®, a groundbreaking broad-spectrum antiviral capable of treating COVID-19, mpox, and dengue
* Hyundai Bioscience presents development updates of its broad-spectrum antiviral at the "Disease Prevention and Control Summit 2024" held in Philadelphia, USA. SEOUL, South Korea, Sept. 11, 2024 /PRNewswire/ -- Dr. Heung-Jeong Woo, a vice president of Hyundai Bioscience shared insights on the...
TTLife Oxygen Concentrator: A VARON-Certified Dealer, Your Reliable Partner in First Aid Preparedness
LOS ANGELES, Sept. 10, 2024 /PRNewswire/ -- TTLife Oxygen Concentrator,
GC Cell and PT Bifarma Adiluhung sign a licensing agreement for Immuncell-LC to expand access in Indonesia
YONGIN, South Korea, Sept. 10, 2024 /PRNewswire/ -- GC Cell, a leading innovator in cell therapy, has officially announced the execution of a landmark 'Technology Transfer and License Agreement' with PT Bifarma Adiluhung(Bifarma), a premier stem cell therapy company inIndonesia. This strategic pa...
Lunit to Present AI-Analyzed Immune Phenotype Study as Immunotherapy Response Predictor for Advanced Gastric Cancer at ESMO Congress 2024
* Lunit SCOPE IO® demonstrates potential to assess immune phenotype as an AI-powered biomarker for predicting efficacy of Nivolumab plus Chemotherapy in advanced gastric cancer, independent of PD-L1 status SEOUL, South Korea, Sept. 10, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provi...
Ractigen Announces Positive Clinical Data for RAG-17 in ALS-SOD1 Treatment from Investigator-Initiated Trial
NANTONG, China and SUZHOU, China, Sept. 10, 2024 /PRNewswire/ -- Ractigen Therapeutics, a clinical-stage pharmaceutical company dedicated to developing innovative therapies, today announced promising clinical data from an Investigator-Initiated Trial (IIT) of RAG-17, a small interfering RNA (siRN...
I-Mab Presents Positive Uliledlimab Pharmacokinetics Data at 2024 World Conference on Lung Cancer
- Pharmacokinetic/pharmacodynamic (PK/PD) modeling data from three Phase 1 studies providing dosing support for upcoming clinical trials - Exposure-Response (E-R) Analysis showed a positive correlation between uliledlimab concentration and ORR probability in mNSCLC patients - Randomized Phase 2 st...
Updated Data from Phase II Clinical Trial of Iruplinalkib Tablets (Qixinke®) Presented at World Conference on Lung Cancer 2024
JINAN, China, Sept. 10, 2024 /PRNewswire/ -- During the World Conference on Lung Cancer (WCLC) held fromSeptember 7-10, 2024, in San Diego, California, updated findings from the Phase II clinical trial (INTELLECT study) were presented. The study assessed the efficacy and safety of Qilu Pharmaceut...
The 2024 International (Bozhou) TCM Expo Kicked Off
BOZHOU, China, Sept. 10, 2024 /PRNewswire/ -- On the morning of September 9, the 2024 International (Bozhou) TCM Expo and the 40th National (Bozhou) TCM Trade Fair officially kicked off in Bozhou,Anhui Province. In recent years, Bozhou has been enhancing mechanisms for the inheritance, innovatio...
Bambusa Therapeutics Inc. Announces Successful Series Seed Funding to Advance Innovative Bispecific Antibodies for Immunology & Inflammation
BOSTON, Sept. 9, 2024 /PRNewswire/ -- Bambusa Therapeutics Inc., a biotechnology company focused on developing bispecific antibodies for the treatment of immunological and inflammatory (I&I) disorders, announced the successful closing of its Series Seed financing round, raising approximately$15 ...
Lunit Joins Roche's Digital Pathology Open Environment to Advance Cancer Biomarker Testing
* Lunit's AI-powered biomarker, Lunit SCOPE PD-L1 TPS, integrates into Roche's navify® Digital Pathology platform to enhance precision medicine and improve cancer biomarker testing SEOUL, South Korea, Sept. 9, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solution...
Neurophet-NTU Singapore, recognized for outstanding joint research
- Neurophet accelerates global expansion for research and partnership in AI-driven brain image analysis SEOUL, South Korea, Sept. 9, 2024 /PRNewswire/ -- Neurophet, an artificial intelligence (AI) solution company for brain disease, announced that Neurophet and NTU (Nanyang Technological Univers...
GenAssist Ltd Announced the First DMD Patient Dosed with its Base Editing Drug
SUZHOU, China, Sept. 9, 2024 /PRNewswire/ -- On September 06, 2024, GenAssist Ltd (GenAssist), announced the first DMD patient dosed with its base editing drug, GEN6050X injection, in an investigator-initiated trial (IIT). "This is the first-in-human trial for DMD gene editing therapy. It marks t...
MGI Launches New Nanopore Sequencing Products with Advanced CycloneSEQ Technology
SHENZHEN, China, Sept. 9, 2024 /PRNewswire/ -- MGI Tech Co., Ltd. ("MGI"), a company committed to building core tools and technologies that drive innovation in life science, today announced the global rights to commercialize and distribute the new sequencing products CycloneSEQ-WT02* and CycloneS...
Formosa Pharmaceuticals Makes First Shipment of APP13007 (Clobetasol Propionate Ophthalmic Suspension, 0.05%) to the United States for Commercialization
TAIPEI, Sept. 9, 2024 /PRNewswire/ -- Formosa Pharmaceuticals, Inc. (hereinafter referred to as "Formosa Pharma," 6838.TW) announced the successful first shipment tothe United States of its new ophthalmic drug, Clobetasol Propionate Ophthalmic Suspension, 0.05% (APP13007), manufactured by Bora P...
Senhwa Biosciences Presents Clinical Data Abstract on Pidnarulex at 2024 ESMO Congress
* The study involved end-stage oncology patients with no other suitable treatment options. * In this Phase Ib study, Pidnarulex (CX-5461) demonstrated acceptable clinical tolerability and showed preliminary signs of efficacy, even in patients who had previously failed treatment with PARP inhi...
Antennova to Present Latest Data of ATN-037 in a Mini Oral Presentation at ESMO Congress 2024
BOSTON, Sept. 8, 2024 /PRNewswire/ -- Antennova, a clinical-stage biotech company focused on oncology today announced thatit will present the latest data of CD73 small molecule inhibitor ATN-037 in a Mini Oral presentation at the 2024 European Society of Medical Oncology Congress (ESMO Congress 2...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00