Medical/Pharmaceuticals
Perioperative Ivonescimab (PD-1/VEGF) for NSCLC Demonstrated Clinically Significant Phase II Results, Presented in Oral Session at WCLC 2024
HONG KONG, Sept. 8, 2024 /PRNewswire/ -- Akeso (9926. HK) announced that its internally developed PD-1/VEGF bispecific antibody ivonescimab showed clinically significant results from a Phase II study, either as a monotherapy or in combination with chemotherapy, for the perioperative treatment of ...
Bc Babycare Marks a Stellar Debut at Germany's Kind+Jugend 2024
COLOGNE, Germany, Sept. 6, 2024 /PRNewswire/ -- Bc Babycare, a leader in innovative products for mothers and babies, made a remarkable debut at Kind+Jugend 2024, the premier international trade fair for baby and toddler outfitting, which opened inCologne, Germany, on September 3. The brand capti...
Akeso to Present Data from 13 Clinical Studies at ESMO 2024, Featuring its Internally Developed Cadonilimab, Ivonescimab, Ligufalimab, and other I/O Antibodies
HONG KONG, Sept. 5, 2024 /PRNewswire/ -- Akeso, Inc. (9926.HK) will showcase promising results from 13 clinical studies on its internally developed PD-1/CTLA-4 bispecific antibody cadonilimab, PD-1/VEGF bispecific antibody ivonescimab, next-generation CD47 monoclonal antibody ligufalimab, and co...
Baird Medical and ExcelFin Acquisition Corp Announce Effectiveness of Registration Statement
FORT MILL, S.C., Sept. 5, 2024 /PRNewswire/ -- Betters Medical Investment
Holdings Limited ("Baird Medical" or the "Company"), a leading microwave
ablation ("MWA") medical device developer and provider inChina, and ExcelFin
Acquisition Corp. ("ExcelFin") (NASDAQ:XFIN
A Small Step towards a Big Mission! World's First! UltraDx Received First Clinical Approval of Single-Molecule Analyzer, in China
SHANGHAI, Sept. 5, 2024 /PRNewswire/ -- UltraDx Bio is proud to announce that its flagship product, the UD-X™ Fully Automated Single-Molecule Array Fluorescence Immunoassay Analyzer, has received its first clinical registration approval inChina. This groundbreaking achievement is now officially l...
Medera Inc. to be Listed on NASDAQ Through a Merger Agreement with Keen Vision Acquisition Corporation
* Medera is a clinical-stage biotechnology company focused on targeting difficult-to-treat cardiovascular diseases using a range of next-generation gene- and cell-based approaches in combination with bioengineered human mini-heart drug discovery and screening technology platforms * Transactio...
Breakthrough Therapy designation for Sanbexin sublingual tablets granted by the United States Food and Drug Administration
NANJING, China, Sept. 5, 2024 /PRNewswire/ -- On September 2, 2024, Simcere Pharmaceuticals Group Ltd. (2096.HK) announced that Sanbexin Sublingual Tablets (Edaravone and Dexborneol sublingual tablets), an innovative drug for stroke, has been granted the Breakthrough Therapy designation by the U....
Grand launch | Sanyou Bio monkeypox antibody made a great breakthrough
SHANGHAI, Sept. 4, 2024 /PRNewswire/ -- On August 28, 2024, Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. announced the launch of 65 kinds of monkeypox antibodies, antigens and cell lines, which has won wide praise from the public. Monkeypox human antibody, as the core product launched by Sanyo...
LakeShore Biopharma Announces Leadership Transitions
GAITHERSBURG, Md., Sept. 4, 2024 /PRNewswire/ -- LakeShore Biopharma Co., Ltd. (Nasdaq: LSB) ("LakeShore Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for ...
HKBU develops Chinese medicine for ulcerative colitis approved by National Medical Products Administration for clinical trial
HONG KONG, Sept. 4, 2024 /PRNewswire/ -- The Centre for Chinese Herbal Medicine Drug Development (CDD) at Hong KongBaptist University (HKBU) has achieved a significant milestone in developing a novel Chinese herbal formulation for ulcerative colitis remission maintenance. Following a submission o...
Immune Phenotyping Identified as Promising Predictive Biomarker by Lunit AI in Biliary Tract Cancer - New publication in CCR
* AI-based tumor microenvironment classification utilizing Lunit SCOPE IO® shows promise for guiding treatment decisions in advanced BTC SEOUL, South Korea ,Sept. 3, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, to...
Biostar Announces Completion of Patient Recruitment for US Phase 1 Clinical Study of Utidelone Capsule
SAN FRANCISCO, Sept. 3, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the US subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the discovery, development and commercialization of innovative oncology drugs, is pleased to announce...
Skyline Therapeutics' Novel Gene Therapy SKG1108 Receives FDA Orphan Drug Designation for Retinitis Pigmentosa
BOSTON and SHANGHAI, Sept. 3, 2024 /PRNewswire/ -- Skyline Therapeutics, an innovation-driven gene therapy company committed to developing unique and novel solutions for rare and severe diseases, announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) fo...
GC Biopharma and Hanmi Pharmaceutical Receives IND Clearance for Phase 1/2 Clinical Trial from the U.S. FDA
* Co-development of innovative new drug for the treatment of Fabry disease as "the world's first once-monthly subcutaneous treatment" * Improves efficacy compared to existing treatment for kidney function, vascular disease, and peripheral nerve disorders YONGIN, South Korea, Sept. 3, 2024 /PR...
Caliway Announces the Initiation of Subject Recruitment in CBL-514 Phase 2b Study for Dercum's Disease
NEW TAIPEI CITY, Sept. 3, 2024 /PRNewswire/ -- Caliway Biopharmaceuticals (Caliway) announced that the subject recruitment of CBL-514 Phase2b study for Dercum's disease (CBL-0202 DD Phase2b study, NCT06303570) has been initiated. The study results are anticipated in Q4 2025. CBL-0202DD study i...
Simcere Zaiming collaborates with TargetRx to introduce a third-generation ALK inhibitor
NANJING, China, Sept. 2, 2024 /PRNewswire/ -- On September 02, 2024, Simcere Zaiming, an innovative oncology company under Simcere Pharmaceutical Group (2096.HK), announced a collaboration agreement with Shenzhen TargetRx Inc. The partnership focuses on the ALK/ROS1 dual receptor tyrosine kinase ...
Jacobio Out-licensed KRAS G12C Inhibitor Glecirasib and SHP2 Inhibitor JAB-3312 to Allist in China
BEIJING and SHANGHAI and BOSTON, Aug. 30, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, today announced that it has granted theChina rights (including mainland China, Hong Kong, Macau, and Taiwan) of KRAS G12C inhibitor glecirasi...
Breaking News | Sanyou Bio Launches Comprehensive Monkeypox Product Line
SHANGHAI, Aug. 30, 2024 /PRNewswire/ -- On August 28, 2024, Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. announced the launch of a comprehensive product line targeting monkeypox, which includes antigens, monoclonal antibodies, and overexpression cell lines. This product line features 65 items ...
/DISREGARD RELEASE: Celltrion USA/
We are advised by Celltrion USA that journalists and other readers should disregard the news release, CelltrionUSA partners with Express Scripts and Cigna Healthcare to expand access to ZYMFENTRA® (infliximab-dyyb), the first and only FDA-approved subcutaneous infliximab on their medical benefit ...
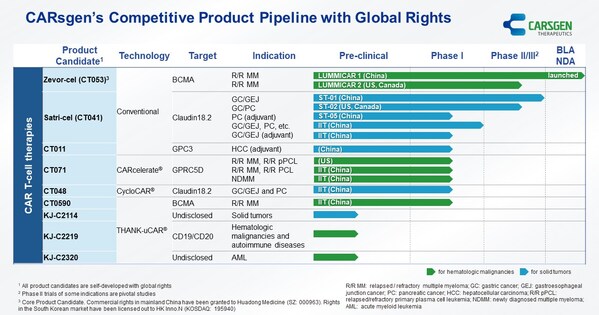
CARsgen® Announces 2024 Interim Results
SHANGHAI, Aug. 29, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, has announced its 2024 Interim Results. Business Highlights * Zevor-cel was...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00