Medical/Pharmaceuticals
Qilian International Holding Group Ltd. Announces Trading Ticker Symbol to "BGM"
CHENGDU, China, Aug. 13, 2024 /PRNewswire/ -- Qilian International Holding Group Ltd. ("Qilian" or the "Company") (NASDAQ: QLI), aChina-based pharmaceutical and chemical products manufacturer, announced today that effective onAugust 11, 2024, its Class A ordinary shares will begin trading on the...
Celltrion USA announces incorporation of adalimumab-aaty, a Humira® biosimilar, to the Costco Member Prescription Program
* Adalimumab-aaty was first approved by the Food and Drug Administration on May 23, 2023 and became commercially available among key distributors across the U.S. onJuly 2, 2023 * Adalimumab-aaty's inclusion creates greater accessibility to treatments for Americans with inflammatory conditions...
Mabwell's Novel Nectin-4 Targeting ADC 9MW2821 Granted Breakthrough Therapy Designation by China's NMPA
SHANGHAI, Aug. 12, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced its novel Nectin-4 targeting ADC (R&D code: 9MW2821) has been granted Breakthrough Therapy Designation (BTD) bythe Center for Drug Evaluation (CDE) of China's...
VISEN Pharmaceuticals announced that the Phase 3 PaTHway China Trial of Palopegteriparatide achieved primary and key secondary endpoints in the treatment of adults with hypoparathyroidism
* 77.6% of patients treated with palopegteriparatide in the PaTHway China Trial achieved the primarymulti-component endpoint compared to 0.0% for placebo (p <0.0001) * Palopegteriparatide was generally safe and well-tolerated, with no discontinuations related to study drug. SHANGHAI, Aug. 12...
Actinogen announces achievement of clinically and statistically significant superiority of Xanamem® over placebo on depression in XanaCIDD phase 2a trial
There was a clinically meaningful and persistent improvement depression measured by the key secondary endpoint of MADRS.[1] The primary endpoint of superiority to placebo in a cognitive "attention composite" of three Cogstate computerized tests was not met with large improvements seen in both Xan...
Milestone: Renal Nerve Mapping / Selective Renal Denervation (msRDN) System by SyMap Medical Ltd Approved for Treatment of Uncontrolled Hypertension in China
SUZHOU, China, Aug. 12, 2024 /PRNewswire/ -- On August 6, 2024, the msRDN System (SyMapCath I®/ SYMPIONEER S1®), developed by Suzhou/China-based SyMap Medical Ltd. (SyMap Medical), was approved by the National Medical Products Administration (NMPA) ofChina and granted a Class III Medical Devices ...
IASO Bio Receives U.S. FDA Approval of Investigational New Drug Application for Equecabtagene Autoleucel for Two New Autoimmune Disease Indications
SHANGHAI, NANJING, China and SAN JOSE, Calif., Aug. 12, 2024 /PRNewswire/ -- IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, today announced that the investigation...
T-MAXIMUM PHARMACEUTICAL Announces Latest Clinical Advances in Allogeneic CAR-T Cell Therapy Breakthrough for Solid Tumors
Summary * T-Maximum Pharmaceutical develops allogeneic CAR-T therapies with a unique CRISPR/Cas9 Gene-Editing platform for solid tumors, provide a solution of two major pain points, GvHD and HvG, aiming to revolutionize cancer. * MT027, the product of target B7H3 received US FDA orphan drug d...
WuXi Biologics and Medigene Enter into a Research Collaboration for Off-the-Shelf TCR-Guided T Cell Engagers
* Partnership leverages Medigene's leadership for T cell receptor (TCR) generation and characterization and WuXi Biologics' unique anti-CD3 mAb, its T cell engager (TCE) platform and proprietary bispecific antibody platform WuXiBody™ * Collaboration is a three-year and potentially multi-TCR p...
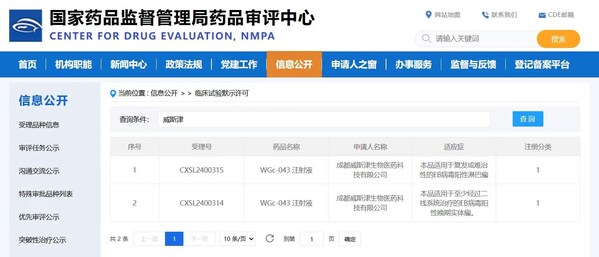
WestGene to Advance Clinical Trials Following Dual IND Approvals for World's First EB Virus-Related mRNA Therapeutic Cancer Vaccine
CHENGDU, China, Aug. 7, 2024 /PRNewswire/ -- WestGene Biopharma is proud to announce that its mRNA therapeutic cancer vaccine, WGc-043, has received dual IND approvals fromChina's National Medical Products Administration (NMPA) and the US FDA. This unprecedented achievement marks the world's firs...
Concord Medical Regains Compliance with NYSE Minimum Price Requirement
BEIJING, Aug. 7, 2024 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specialized in cancer treatment, research, education and prevention inChina, today announced that it has received a letter from the New York Stoc...
Formosa Pharma and Eyenovia Announce Initiation of Co-Development of Clobetasol Propionate Ophthalmic Suspension, 0.05%, for the treatment of Acute Dry Eye Disease in United States
TAIPEI, Aug. 7, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals ("Formosa", 6838.TWO) announced today that the company has signed a non-binding terms agreement with Eyenovia, Inc. ("Eyenovia", NASDAQ: EYEN), whereby the companies will co-develop Clobetasol Propionate Ophthalmic Suspensi...
I-Mab Appoints U.S. Auditor, PricewaterhouseCoopers LLP (PwC)
Engagement is part of I-Mab's commitment to transition to a U.S.-based biotech PwC to serve as independent registered public accounting firm for FY 2024 ROCKVILLE, Md., Aug. 7, 2024 /PRNewswire/ -- I-Mab (NASDAQ: IMAB) ("I-Mab", the "Company"), a U.S.-based, global biotech company, exclusively f...
Senhwa Biosciences receives US FDA Study May Proceed letter for the Phase I/II study of Silmitasertib (CX-4945) in combination with chemotherapy in children and young adults with relapsed refractory solid tumors
TAIPEI and SAN DIEGO, Aug. 6, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced receipt of a "Study May Proceed" letter from the U.S. Food and Drug A...
Datasea's Digital Technology Subsidiary Selected as Prospective Partner for Future Projects with Subsidiary of China Mobile
The Prospective Partnership with a Subsidiary of China Mobile, one of the World's Largest Mobile Operator, Underscores DTSS' Achievements in 5G-AI Communications BEIJING, Aug. 6, 2024 /PRNewswire/ -- Datasea Inc. (Nasdaq: DTSS) ("Datasea" or the "Company"), a digital technology company incorpora...
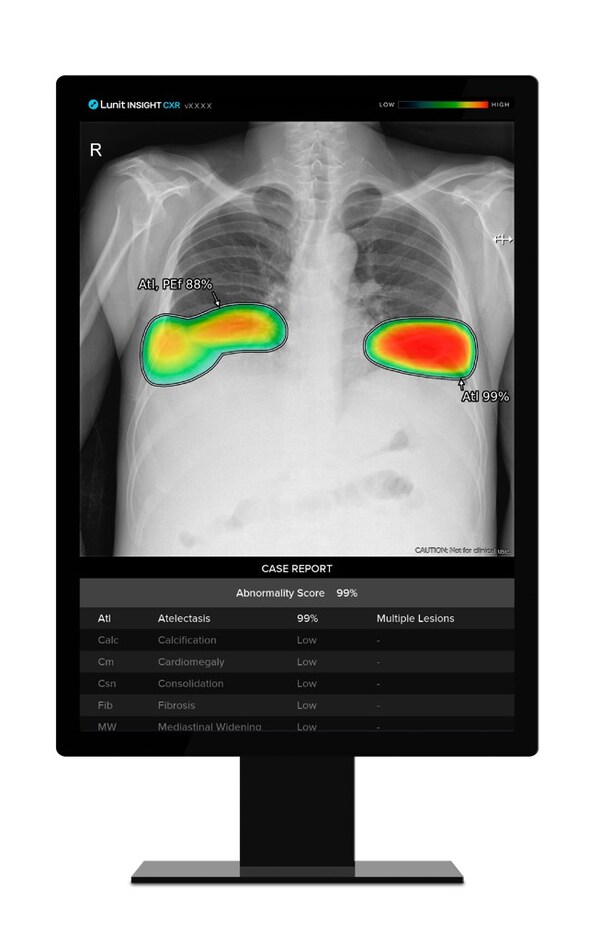
Lunit's AI for Tuberculosis Detection Tops in 12-Product Comparative Study
* Lancet Digital Health study highlights Lunit INSIGHT CXR as top performer in TB screening, showing promise for improving detection in high-burden settings SEOUL, South Korea, Aug. 6, 2024 /PRNewswire/ -- Lunit, a leading provider of AI-powered solutions for cancer diagnostics and therapeutics,...
Samsung Biologics joins the Pharmaceutical Supply Chain Initiative as Supplier Partner
* Samsung Biologics to embed PSCI principles into business practices for responsible value chain management * Supplier Partnership reaffirms company's commitment to decarbonize and build resilient supply chains INCHEON, South Korea, Aug. 6, 2024 /PRNewswire/ -- Samsung Biologics (KRX: 207940....
First Patient Enrolled in the US Phase 2 Combination Therapy of Akeso's Ligufalimab with Azacitidine for Myelodysplastic Syndrome
HONG KONG, Aug. 6, 2024 /PRNewswire/ -- Akeso has announced the completion of the first patient enrollment in the US for the phase II clinical trial of its innovative CD47 monoclonal antibody, ligufalimab (AK117), in combination with azacitidine for patients with newly diagnosed higher-risk myelo...
OBiO Technology Congratulates on The First Clinical Gene Editing Therapy to Treat An Overseas Patient in China by CorrectSequence Therapeutics
SHANGHAI, Aug. 5, 2024 /PRNewswire/ -- Recently, CorrectSequence Therapeutics Co., Ltd. (Correctseq) announced a significant milestone in their base editing therapy CS-101 for transfusion-dependent β-thalassemia. Utilizing their pioneering transformer Base Editor (tBE), Correctseq has successfull...
META Pharmaceuticals announces FDA Grants Rare Pediatric Disease Designation to META-001-PH for the Treatment of Primary Hyperoxaluria
HONG KONG, Aug. 5, 2024 /PRNewswire/ -- META Pharmaceuticals Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation (RPDD) to its investigational new drug META-001-PH for the treatment of primary hyperoxaluria (PH).Primary hyperoxaluria is a...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00