Medical/Pharmaceuticals
IASO Bio Receives U.S. FDA Approval of Investigational New Drug Application for Equecabtagene Autoleucel for Two New Autoimmune Disease Indications
SHANGHAI, NANJING, China and SAN JOSE, Calif., Aug. 12, 2024 /PRNewswire/ -- IASO Biotherapeutics ("IASO Bio"), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, today announced that the investigation...
T-MAXIMUM PHARMACEUTICAL Announces Latest Clinical Advances in Allogeneic CAR-T Cell Therapy Breakthrough for Solid Tumors
Summary * T-Maximum Pharmaceutical develops allogeneic CAR-T therapies with a unique CRISPR/Cas9 Gene-Editing platform for solid tumors, provide a solution of two major pain points, GvHD and HvG, aiming to revolutionize cancer. * MT027, the product of target B7H3 received US FDA orphan drug d...
WuXi Biologics and Medigene Enter into a Research Collaboration for Off-the-Shelf TCR-Guided T Cell Engagers
* Partnership leverages Medigene's leadership for T cell receptor (TCR) generation and characterization and WuXi Biologics' unique anti-CD3 mAb, its T cell engager (TCE) platform and proprietary bispecific antibody platform WuXiBody™ * Collaboration is a three-year and potentially multi-TCR p...
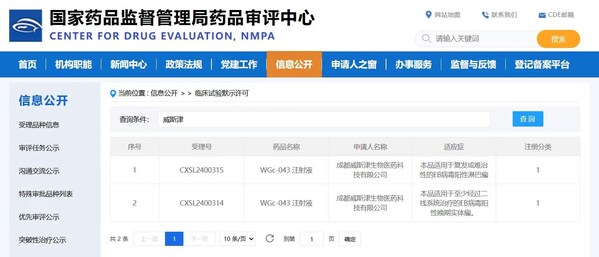
WestGene to Advance Clinical Trials Following Dual IND Approvals for World's First EB Virus-Related mRNA Therapeutic Cancer Vaccine
CHENGDU, China, Aug. 7, 2024 /PRNewswire/ -- WestGene Biopharma is proud to announce that its mRNA therapeutic cancer vaccine, WGc-043, has received dual IND approvals fromChina's National Medical Products Administration (NMPA) and the US FDA. This unprecedented achievement marks the world's firs...
Concord Medical Regains Compliance with NYSE Minimum Price Requirement
BEIJING, Aug. 7, 2024 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specialized in cancer treatment, research, education and prevention inChina, today announced that it has received a letter from the New York Stoc...
Formosa Pharma and Eyenovia Announce Initiation of Co-Development of Clobetasol Propionate Ophthalmic Suspension, 0.05%, for the treatment of Acute Dry Eye Disease in United States
TAIPEI, Aug. 7, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals ("Formosa", 6838.TWO) announced today that the company has signed a non-binding terms agreement with Eyenovia, Inc. ("Eyenovia", NASDAQ: EYEN), whereby the companies will co-develop Clobetasol Propionate Ophthalmic Suspensi...
I-Mab Appoints U.S. Auditor, PricewaterhouseCoopers LLP (PwC)
Engagement is part of I-Mab's commitment to transition to a U.S.-based biotech PwC to serve as independent registered public accounting firm for FY 2024 ROCKVILLE, Md., Aug. 7, 2024 /PRNewswire/ -- I-Mab (NASDAQ: IMAB) ("I-Mab", the "Company"), a U.S.-based, global biotech company, exclusively f...
Senhwa Biosciences receives US FDA Study May Proceed letter for the Phase I/II study of Silmitasertib (CX-4945) in combination with chemotherapy in children and young adults with relapsed refractory solid tumors
TAIPEI and SAN DIEGO, Aug. 6, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced receipt of a "Study May Proceed" letter from the U.S. Food and Drug A...
Datasea's Digital Technology Subsidiary Selected as Prospective Partner for Future Projects with Subsidiary of China Mobile
The Prospective Partnership with a Subsidiary of China Mobile, one of the World's Largest Mobile Operator, Underscores DTSS' Achievements in 5G-AI Communications BEIJING, Aug. 6, 2024 /PRNewswire/ -- Datasea Inc. (Nasdaq: DTSS) ("Datasea" or the "Company"), a digital technology company incorpora...
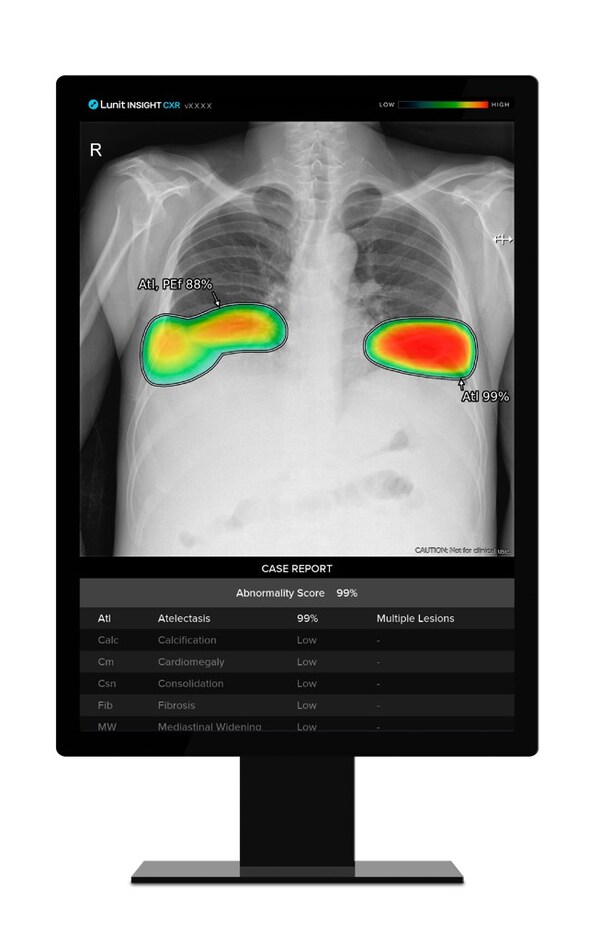
Lunit's AI for Tuberculosis Detection Tops in 12-Product Comparative Study
* Lancet Digital Health study highlights Lunit INSIGHT CXR as top performer in TB screening, showing promise for improving detection in high-burden settings SEOUL, South Korea, Aug. 6, 2024 /PRNewswire/ -- Lunit, a leading provider of AI-powered solutions for cancer diagnostics and therapeutics,...
Samsung Biologics joins the Pharmaceutical Supply Chain Initiative as Supplier Partner
* Samsung Biologics to embed PSCI principles into business practices for responsible value chain management * Supplier Partnership reaffirms company's commitment to decarbonize and build resilient supply chains INCHEON, South Korea, Aug. 6, 2024 /PRNewswire/ -- Samsung Biologics (KRX: 207940....
First Patient Enrolled in the US Phase 2 Combination Therapy of Akeso's Ligufalimab with Azacitidine for Myelodysplastic Syndrome
HONG KONG, Aug. 6, 2024 /PRNewswire/ -- Akeso has announced the completion of the first patient enrollment in the US for the phase II clinical trial of its innovative CD47 monoclonal antibody, ligufalimab (AK117), in combination with azacitidine for patients with newly diagnosed higher-risk myelo...
OBiO Technology Congratulates on The First Clinical Gene Editing Therapy to Treat An Overseas Patient in China by CorrectSequence Therapeutics
SHANGHAI, Aug. 5, 2024 /PRNewswire/ -- Recently, CorrectSequence Therapeutics Co., Ltd. (Correctseq) announced a significant milestone in their base editing therapy CS-101 for transfusion-dependent β-thalassemia. Utilizing their pioneering transformer Base Editor (tBE), Correctseq has successfull...
META Pharmaceuticals announces FDA Grants Rare Pediatric Disease Designation to META-001-PH for the Treatment of Primary Hyperoxaluria
HONG KONG, Aug. 5, 2024 /PRNewswire/ -- META Pharmaceuticals Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Rare Pediatric Disease Designation (RPDD) to its investigational new drug META-001-PH for the treatment of primary hyperoxaluria (PH).Primary hyperoxaluria is a...
Antennova Announces CD73 Small Molecule Inhibitor Accepted for Mini Oral Presentation at ESMO Congress 2024
BOSTON, Aug. 5, 2024 /PRNewswire/ -- Antennova, a clinical-stage biotech company focused on oncology today announced that the orally administered CD73 small molecule inhibitor ATN-037(also known as ATG-037) has been accepted for Mini Oral presentation at the 2024 European Society of Medical Oncol...
QBIOTICS WELCOMES STEPHEN DOYLE AS CHIEF EXECUTIVE OFFICER
* Stephen Doyle has more than 24 years of experience in the global pharmaceutical industry, including leadership positions with companies such as Sanofi Aventis and Boehringer Ingelheim. He was most recently Chief Business Officer at Aslan Pharmaceuticals. * Mr Doyle brings extensive knowledg...
So-Young to Report Second Quarter 2024 Financial Results on August 23, 2024
BEIJING, Aug. 5, 2024 /PRNewswire/ -- So-Young International Inc. (NASDAQ: SY) ("So-Young" or the "Company"), the largest and most vibrant social community in China for consumers, professionals and service providers in the medical aesthetics industry, today announced that it will report its financ...
Formosa Pharmaceuticals Announces Licensing Agreement with Apotex Inc., for Commercialization of Clobetasol Propionate Ophthalmic Suspension for Ocular Surgery Relief and Recovery
TAIPEI, Aug. 5, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals (" Formosa", 6838.TWO) announced today that the company has entered into an exclusive licensing agreement with Apotex Inc. ("Apotex"), for exclusive rights inCanada to the commercialization of clobetasol propionate ophthalmi...
INAUGURAL NUHS SCIENTIFIC & INNOVATION SUMMIT CHAMPIONS PREDICTIVE, PRECISE AND PERSONALISED CARE
From digital twin technology to leveraging AI in tackling obesity, the NUHS Scientific & Innovation Summit is a congregation of the brightest minds in cutting-edge medicine – with patients' utmost care at heart SINGAPORE, Aug. 3, 2024 /PRNewswire/ -- Hepatocellular carcinoma (HCC) is one of the ...
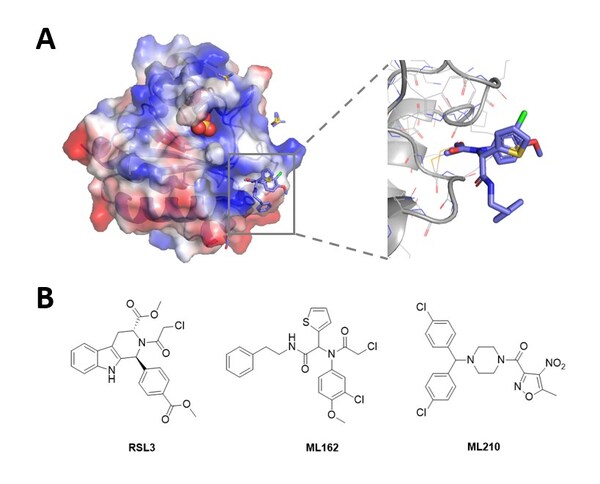
Novel Non-Covalent Hits Against GPX4 Identified Using the RiDYMO® Reinforced Dynamics Platform of DP Technology
Research on ferroptosis is gaining momentum, but the development of small molecule inhibitors faces numerous challenges BEIJING, Aug. 1, 2024 /PRNewswire/ -- Glutathione peroxidase 4 (GPX4) is recognized as a critical regulator of ferroptosis, playing a significant role in lipid and amino acid m...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 328 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 310 media titles]
2026-02-26 19:57HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 309 media titles]
2026-02-26 09:30Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13