Medical/Pharmaceuticals
Biostar Announces Completion of Patient Recruitment for US Phase 1 Clinical Study of Utidelone Capsule
SAN FRANCISCO, Sept. 3, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the US subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the discovery, development and commercialization of innovative oncology drugs, is pleased to announce...
Skyline Therapeutics' Novel Gene Therapy SKG1108 Receives FDA Orphan Drug Designation for Retinitis Pigmentosa
BOSTON and SHANGHAI, Sept. 3, 2024 /PRNewswire/ -- Skyline Therapeutics, an innovation-driven gene therapy company committed to developing unique and novel solutions for rare and severe diseases, announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) fo...
GC Biopharma and Hanmi Pharmaceutical Receives IND Clearance for Phase 1/2 Clinical Trial from the U.S. FDA
* Co-development of innovative new drug for the treatment of Fabry disease as "the world's first once-monthly subcutaneous treatment" * Improves efficacy compared to existing treatment for kidney function, vascular disease, and peripheral nerve disorders YONGIN, South Korea, Sept. 3, 2024 /PR...
Caliway Announces the Initiation of Subject Recruitment in CBL-514 Phase 2b Study for Dercum's Disease
NEW TAIPEI CITY, Sept. 3, 2024 /PRNewswire/ -- Caliway Biopharmaceuticals (Caliway) announced that the subject recruitment of CBL-514 Phase2b study for Dercum's disease (CBL-0202 DD Phase2b study, NCT06303570) has been initiated. The study results are anticipated in Q4 2025. CBL-0202DD study i...
Simcere Zaiming collaborates with TargetRx to introduce a third-generation ALK inhibitor
NANJING, China, Sept. 2, 2024 /PRNewswire/ -- On September 02, 2024, Simcere Zaiming, an innovative oncology company under Simcere Pharmaceutical Group (2096.HK), announced a collaboration agreement with Shenzhen TargetRx Inc. The partnership focuses on the ALK/ROS1 dual receptor tyrosine kinase ...
Jacobio Out-licensed KRAS G12C Inhibitor Glecirasib and SHP2 Inhibitor JAB-3312 to Allist in China
BEIJING and SHANGHAI and BOSTON, Aug. 30, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, today announced that it has granted theChina rights (including mainland China, Hong Kong, Macau, and Taiwan) of KRAS G12C inhibitor glecirasi...
Breaking News | Sanyou Bio Launches Comprehensive Monkeypox Product Line
SHANGHAI, Aug. 30, 2024 /PRNewswire/ -- On August 28, 2024, Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. announced the launch of a comprehensive product line targeting monkeypox, which includes antigens, monoclonal antibodies, and overexpression cell lines. This product line features 65 items ...
/DISREGARD RELEASE: Celltrion USA/
We are advised by Celltrion USA that journalists and other readers should disregard the news release, CelltrionUSA partners with Express Scripts and Cigna Healthcare to expand access to ZYMFENTRA® (infliximab-dyyb), the first and only FDA-approved subcutaneous infliximab on their medical benefit ...
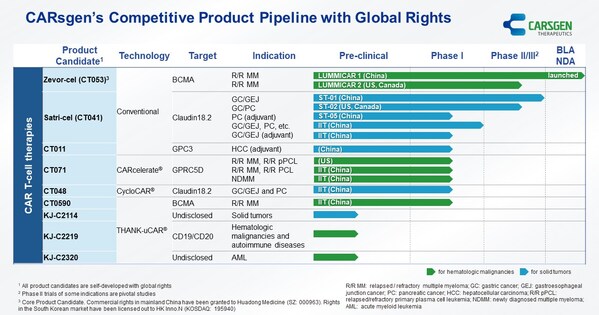
CARsgen® Announces 2024 Interim Results
SHANGHAI, Aug. 29, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, has announced its 2024 Interim Results. Business Highlights * Zevor-cel was...
Medicilon Expands Global Presence with Official Opening of Second R&D Center in Massachusetts
BOSTON, Aug. 29, 2024 /PRNewswire/ -- In August 2024, Medicilon USA Corp (Medicilon) officially commenced operations at its second R&D center in Massachusetts, USA. Research at the new facility will focus on animal models for PK/PD and toxicology studies. This milestone heralds Medicilon's continu...
Transcenta Holding Limited (Stock Code: 6628.HK) Announce 2024 Interim Results
2024 Interim Result Highlights (For the six months ended June 30, 2024) * Company published the efficacy and safety data of Cohort-G of TranStar102 study for osemitamab (TST001), plus checkpoint inhibitor and CAPOX as the first-line treatment of patients with locally advanced or metastatic G/GE...
Akeso's 2024 First Half Interim Results: Expanding Global Lead in IO Bispecific Antibodies, Advancing New Therapies, and Accelerating Commercialization
* Approval of ivonescimab for 2L+ EGFRm NSCL; HARMONi-A is the only phase III study that demonstrates significant benefit across all subgroups for PFS, and is also the only study to achieve the primary endpoint while showing a positive trend in OS benefit; HARMONi-A study presented in an oral p...
Asieris Pharmaceuticals Releases 2024 Semi-Annual Report, Highlighting Steady Progress in Launching Blockbuster Product for Precancerous Cervical Lesions and Continued Strengthening of Commercial Revenue Generation Capabilities
As of the date of this publication, APL-1702 has not been approved for the treatment of cervical high-grade squamous intraepithelial lesions (HSIL); APL-1706 has not been approved for the diagnosis or surgical treatment of bladder cancer inChina. This article is intended to disclose the latest d...
Jenscare, with innovative TTVR, releases 2024H1 interim results
BEIJING, Aug. 28, 2024 /PRNewswire/ -- Jenscare Scientific Co., Ltd. ("Jenscare" or the "Company") (HKEX: 9877), an innovative medical device company dedicated to interventional treatment for structural heart diseases with TTVR breakthroughs, released interim results for 2024H1 endedJune 30, 2024...
Tigermed Reports 2024 Interim Results
HANGZHOU, China, Aug. 28, 2024 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. ("Tigermed" or the "company") (Stock code: 300347.SZ / 3347.HK), a leading provider of clinical research solutions across full lifecycle of global biopharmaceutical and medical device products, announced its int...
Fosun Pharma Announces 2024 Interim Results
Breakthroughs for Innovative Products Going Overseas with Global Operation Capabilities Continuing to Strengthen SHANGHAI, Aug. 28, 2024 /PRNewswire/ -- On August 27, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma" or "the Group"; Stock Code: 600196.SH; 02196.HK), announced its o...
Harbour BioMed Announces 2024 Interim Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 28, 2024 /PRNewswire/ -- Harbour BioMed ("HBM", or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology an...
Hyundai Bioscience to conduct phase 3 clinical trial for high-risk group of COVID-19 patients with the goal of emergency use authorization
* "Xafty® can be the only oral COVID-19 treatment that can be prescribed to high-risk group patients who cannot take Paxlovid®" * "While maintaining the current process for emergency use authorization for mild-to-moderate COVID-19 patients, Hyundai Bioscience will expedite Phase 3 clinical tr...
Achieva Medical Entered into an Exclusive Distribution Agreement with NowYon Medical
SUZHOU, China, Aug. 28, 2024 /PRNewswire/ -- Peijia Medical (HKG:9996), a leading Chinese domestic player in the high-growth transcatheter valve therapeutics and neurovascular interventions markets, announced that its wholly-owned subsidiary, Achieva Medical Limited ("Achieva Medical"), entered ...
Yunovia Announces IND Approval by the MFDS to Initiate Phase 1 MAD Study for the Small Molecule GLP-1 Agonist
SEOUL, South Korea, Aug. 27, 2024 /PRNewswire/ -- Yunovia, a drug R&D subsidiary of Ildong Pharmaceutical Group, announced on the 26th that the Ministry of Food and Drug Safety (MFDS) of Korea has cleared the IND application of Phase 1 Multiple Ascending Dose (MAD) study for ID110521156, an oral...
Week's Top Stories
Most Reposted
Visa partners with Laufey to spread the magic of travel in Asia Pacific; to be Official Payment Partner for Laufey: A Matter of Time Tour
[Picked up by 309 media titles]
2026-03-04 12:35SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15Infobip is set to launch AgentOS to orchestrate autonomous AI-driven customer journeys at scale
[Picked up by 290 media titles]
2026-03-02 09:00Klook's Spring Readiness Index shows how Asia's travelers are preparing for spring travel across Japan, South Korea, and Mainland China
[Picked up by 290 media titles]
2026-03-03 15:49COL and NASDAQ-Listed BeLive Holdings Unveil World's First "Microdrama in a Box" in Headline Hong Kong FILMART 2026 Launch
[Picked up by 286 media titles]
2026-03-05 17:14