Pharmaceuticals
Live from ASCO 2024 | Oral Report Released Latest Data of Olverembatinib in SDH-Deficient GIST, Including a CBR of 92.3%
SUZHOU, China and ROCKVILLE, Md., June 4, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it has released updated clinical data of olvere...
Live from ASCO 2024 | Ascentage Pharma Releases Updated Data Showing Promising Efficacy and Safety of Lisaftoclax in Patients with WM
SUZHOU, China, and ROCKVILLE, Md., June 4, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it has released updated results from a global,...
CARsgen Presents Updated Results on Satri-cel in Nature Medicine and at 2024 ASCO
SHANGHAI, June 4, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that the final follow-up results of the investigator-initiated trial C...
GCBP Sponsors 2024 Spring Symposium for KASBP and Present Research Findings
YONGIN, South Korea, June 4, 2024 /PRNewswire/ -- GC Biopharma announced that it will attend 2024 Spring Symposium of the Korean American Society in Biotech and Pharmaceuticals (KASBP) onJune 7-8th at Waltham, Massachusetts as a sponsor to share keynote presentation, present Korean Scientist Awar...
Live from ASCO 2024 | Updated Data of Bcl-2 Inhibitor Lisaftoclax Combined with Azacitidine in Patients with AML Demonstrate Promising Efficacy and Manageable Safety
SUZHOU, China and ROCKVILLE, Md., June 4, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that it has released the latest results from a Phase...
Fapon Biopharma Announces a Safer Immunotherapy for Cancers
SAN DIEGO, June 3, 2024 /PRNewswire/ -- At Bio International Convention 2024, which is taking place here inSan Diego during June 3-6, Fapon Biopharma, an innovator in developing therapeutic antibodies and fusion proteins, has announced an immunocytokine with modified IL-10M fused to anti-PD-1 ant...
Minghui Pharmaceutical Presents Phase 1/2 Clinical Data of MHB088C (B7-H3 ADC) as Monotherapy for the Treatment of Patients with Recurrent or Metastatic Solid Tumors at the 2024 ASCO Annual Meeting
SHANGHAI, June 3, 2024 /PRNewswire/ -- Minghui Pharmaceutical, Inc., a late-stage clinical biopharmaceutical company, announced today that the preliminary Phase 1/2 clinical data of MHB088C (B7-H3 ADC) was presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting in an ora...
Formosa Laboratories Completes the Acquisition of Synchem to Expand North American CDMO Footprint
TAOYUAN, June 3, 2024 /PRNewswire/ -- Formosa Laboratories, Inc., a leading API supplier and CDMO headquartered inTaiwan, has successfully completed its acquisition of SynChem, Inc., a contract research laboratory located in suburban Chicago. SynChem will now operate under the new name SynChem-For...
Leads Biolabs' Innovative Cancer Treatment LBL-024, an Anti-PDL1/4-1BB Bispecific Antibody Achieved Outstanding Phase II Results, Has Been Presented in Clinical Science Symposium at 2024 ASCO Annual Meeting.
NANJING, China, June 2, 2024 /PRNewswire/ -- The annual meeting of the American Society of Clinical Oncology (ASCO) commenced onMay 31st, Showcasing groundbreaking cancer research from around the world. According to official information, over 7,000 abstracts were submitted this year. After rigoro...
Keymed Biosciences Announces Long-term Efficacy and Safety Data from a Phase III Clinical Trial of Stapokibart for the Treatment of Moderate-to-severe Atopic Dermatitis
CHENGDU, China, June 2, 2024 /PRNewswire/ -- Keymed Biosciences Inc. (HKEX: 02162) today announced the long-term efficacy and safety data of a Phase III clinical trial of stapokibart injection in patients with moderate-to-severe atopic dermatitis (AD) has been released by way of oral presentation...
Jacobio Pharma Presents SHP2 Plus KRAS G12C Data at ASCO
BEIJING and SHANGHAI and CHICAGO, June 1, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, presented updated data of a KRAS G12C inhibitor glecirasib (JAB-21822) in combination with aSHP2 inhibitor (JAB-3312) in frontline non-small ...
GenFleet Therapeutics Announces Efficacy & Safety Result from Phase II Trial for First-line NSCLC Treatment in KROCUS Study, fulzerasib (KRAS G12C Inhibitor) in Combination with cetuximab, in a Late-breaking Abstract at the Oral Presentation of 2024 ASCO Annual Meeting
SHANGHAI and CHICAGO, June 1, 2024 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, today announced the phase II trial data of KROCUS Study, fulzerasib (GFH925, KRAS G12C inhibitor) in combination with cet...
Immunofoco Biotech to Unveil Solid Tumor CAR-T Programs Clinical Trial Data at 2024 ASCO Meeting
CHICAGO, June 1, 2024 /PRNewswire/ -- On June 1st, 2024, Immunofoco Biotech, a company dedicated to developing cell therapy products for solid tumors, announced that the clinical research data for two of its products have been accepted for presentation at the 2024 American Society of Clinical Onc...
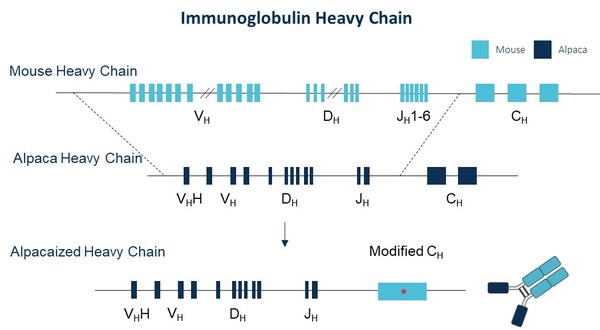
Immunocan Launches Innovative Alpaca-derived Nanobody Discovery Platform: ImmuAlpaca® Mouse
BOSTON, June 1, 2024 /PRNewswire/ -- Immunocan announces significant progress
in the construction of its alpaca-derived nanobody discovery platform,
ImmuAlpaca® mouse. The IGH-VDJ genes in the mouse genome have been successfully
replaced by alpaca IGH-VDJ genes in-situ.
TiumBio Presents Phase 1b Interim Results for TU2218 in Combination with Pembrolizumab at ASCO 2024 Annual Meeting
* Poster presentation features the interim data from an ongoing Phase 1b clinical trial of TU2218 plus Keytruda combination therapy in patients with advanced solid tumors * A 40% overall response rate (ORR) and 100% disease control rate (DCR) were achieved in high-dose cohort (195 mg/day) *...
Ivonescimab in Combination with Chemotherapy Approved in China by NMPA for 2L+ EGFRm NSCLC based on HARMONi-A Clinical Trial: Positive Trend Observed in Overall Survival towards Ivonescimab Plus Chemotherapy
Separate & Distinct from HARMONi-2 Announcement, HARMONi-A Showed Clinically Meaningful and Statistically Significant Benefit: PFS Hazard Ratio of 0.46 For Subset of Patients Previously Receiving 3rd Generation EGFR-TKI: PFS Hazard Ratio of 0.48 5.6% Treatment Discontinuation of Ivonescimab due...
GRIT and Quangang Forge Strategic Partnership to Accelerate Localization of Interleukin-2
SHANGHAI, May 31, 2024 /PRNewswire/ -- Shanghai Grit Biotechnology Co., Ltd. (GRIT) and Shandong Quangang Pharmaceutical Co., Ltd. (Quangang) announced the establishment of a formal strategic partnership to leverage both parties' R&D capabilities in innovative T-cell therapy. The objective is to ...
Neurophet to participate BIO USA… exploring global partnership and business
* Neurophet's AI brain image analysis technology improves time and cost efficiency for drug development * Aims to create business opportunities with global pharmaceutical companies SEOUL, South Korea, May 31, 2024 /PRNewswire/ -- Neurophet, an artificial intelligence (AI) solution company for...
OBiO Technology Officially Launched the Center for Clinical Evaluation and Translation of Advanced Therapies for Pediatric Rare and Genetic Diseases
SHANGHAI, May 30, 2024 /PRNewswire/ -- On May 23, OBiO Technology, a world leading contract development and manufacturing organization for cell and gene therapy, officially launched the "Center for Clinical Evaluation and Translation of Advanced Therapies for Pediatric Rare and Genetic Diseases" ...
Kexing Biopharm Obtained Clinical Trial Approval for its Self-developed Class I Innovative Drug--Long-acting Growth Hormone
SHENZHEN, China, May 30, 2024 /PRNewswire/ -- On the 24th of May, Kexing Biopharm (688136.SH) announced that Shenzhen Kexing Pharmaceutical Co., Ltd., its wholly-owned subsidiary, recently received a Notice of Approval for Drug Clinical Trials from the National Medical Products Administration, ap...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 327 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 308 media titles]
2026-02-26 19:57HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 308 media titles]
2026-02-26 09:30Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13