Pharmaceuticals
Minghui Pharmaceutical to Present the Phase I/II Study of MHB088C (B7-H3 ADC) for the Treatment of Patients with Recurrent or Metastatic Solid Tumors in Late-breaking Oral Presentation at the 2024 ASCO Annual Meeting
SHANGHAI, May 8, 2024 /PRNewswire/ -- Minghui Pharmaceutical, Inc., a late-stage clinical biopharmaceutical company focused on autoimmune diseases and oncology, will feature Dr.Lin Shen from Beijing Cancer Hospital at the upcoming ASCO Annual Meeting inChicago. Dr. Shen will present the results f...
FDA Grants Orphan Drug Designation to 9MW2821
SHANGHAI, May 7, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that its self-developed novel Nectin-4-targeting ADC (R&D code: 9MW2821) has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Adminis...
Meihua International Medical Technologies Co., Ltd. to Present at the Emerging Growth Conference on May 9, 2024
Meihua International Medical Technologies Co., Ltd. invites individual and institutional investors as well as advisors and analysts, to attend its real-time, interactive presentation at the Emerging Growth Conference. YANGZHOU, China, May 7, 2024 /PRNewswire/ -- Meihua International Medical Tech...
GC Genome to Present New Clinical Data on Colorectal Cancer Detection at the ASCO Annual Meeting 2024
YONGIN, South Korea, May 7, 2024 /PRNewswire/ -- GC Genome Corporation, a leading diagnostics company, today announced that it will present the new clinical data of its AI-based liquid biopsy platform on colorectal cancer detection at the 2024 American Society of Clinical Oncology (ASCO) Annual ...
HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
* HanAll has initiated a Phase III VELOS-4 study to evaluate the efficacy and safety of tanfanercept in dry eye based on the findings from the previous Phase III VELOS-3 study. * Tanfanercept demonstrated statistically significant improvement on the secondary outcome measure, Schirmer testing...
Jacobio Pharma Announced its KRAS G12C inhibitor reached the primary endpoint
BEIJING and SHANGHAI and BOSTON, April 30, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced that the data from the Phase II registrational study of the KRAS G12C inhibitor glecirasib were offically reported at the April ASCO Plenary Series, which was held online. Prof. Yuankai Shi, chief ...
Eluminex Biosciences Announces FDA Acceptance of Investigational New Drug (IND) Application for EB-105 - A Novel Trispecific Fusion Antibody for Diabetic Macular Edema (DME) - and Upcoming Scientific Presentations
SAN FRANCISCO and SUZHOU, China, April 30, 2024 /PRNewswire/ -- Eluminex Biosciences (Eluminex), a privately-held biotechnology company focused on the development of advanced protein therapeutics for vision-threatening diseases and dermal facial aesthetics announced the acceptance of their EB-105...
National Medical Products Administration (NMPA) Approves Chipscreen Bioscience's Chidamide (Epidaza) combined with R-CHOP for the treatment of diffuse large B-cell lymphoma
SHENZHEN, China, April 30, 2024 /PRNewswire/ -- Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) announced that the company's lead innovative product Chidamide (Epidaza®) , an oral subtype-selective histone deacetylase (HDAC) inhibitor, combined with R-C...
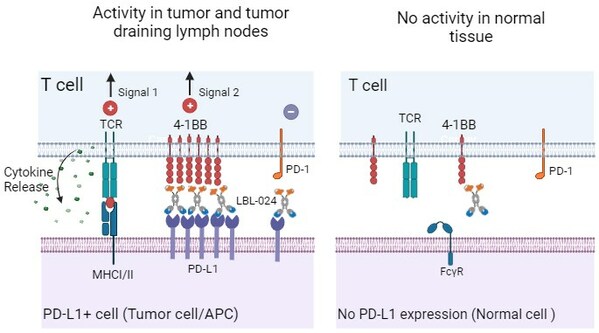
A Potential First-In-Class Drug: CDE Approved Single-Arm Pivotal Clinical Study of LBL-024, An Anti-PD-L1/4-1BB Bispecific Antibody Developed by Leads Biolabs
NANJING, China, April 30, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to co...
HanAll Biopharma Reports Q1 2024 Financial Results and Provides Business Update
* Delivered solid performance to start 2024, with record-breaking first quarter revenue of34.1 billion KRW. Strong sales momentum continued from key products, funding investments in ongoing R&D programs. * Phase 3 VELOS-4 study of tanfanercept in dry eye disease expected to be initiated in th...
I-MAB Filed 2023 Annual Report on Form 20-F
ROCKVILLE, Md., April 30, 2024 /PRNewswire/ -- I-Mab (the "Company") (NASDAQ: IMAB), a U.S.-based, global biotech company, exclusively focused on the development and potential commercialization of highly differentiated immunotherapies for the treatment of cancer, today announced that it has filed...
111 to Announce First Quarter 2024 Unaudited Financial Results on May 23, 2024 - Conference Call to Follow
SHANGHAI, April 30, 2024 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced that it will report its unaudited financial result...
WuXi Biologics Releases 2023 ESG Report Demonstrating Strong Sustainability Commitment
* The Company demonstrated a deep commitment to achieving ESG success in partnership with global clients, creating long-term value for all stakeholders. * The Company made remarkable progress in tackling climate change, achieving a 29% intensity reduction of Scope 1 and Scope 2 greenhouse gas ...
Technoderma Medicines Advances TDM-180935 Atopic Dermatitis Clinical Program with Phase 2 Trial
CHENGDU, China, April 29, 2024 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report the Company has begun dosing patients in its Phase 2a clinical trial (NCT06363461) of topical TDM-180935 ointment. This clinical trial in t...
YS Biopharma Receives Additional 180 Day Extension by Nasdaq to Regain Compliance with Minimum Bid Price Rule
GAITHERSBURG, Md., April 29, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious dise...
Prota Therapeutics' CEO Mimi Tang honoured at BioMelbourne Network's Women in Leadership Awards 2024
MELBOURNE, Australia, April 29, 2024 /PRNewswire/ -- Prota Therapeutics' CEO ProfessorMimi Tang has been honoured in the prestigious 2024 BioMelbourne Network Women in Leadership Awards. Professor Tang's exceptional work as a lead researcher for the Murdoch Children's Research Institute (MCRI) an...
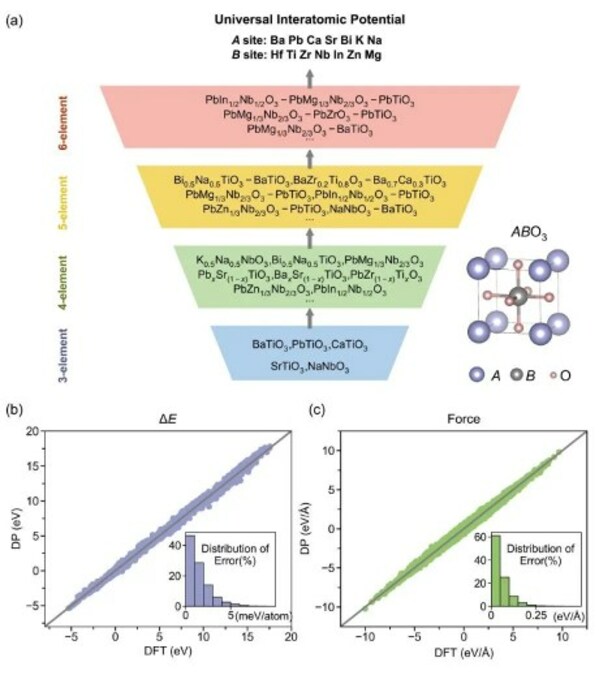
DP Technology DevDay 2024 Showcases Large Science Models and Announces Open Science Initiative
BEIJING, April 29, 2024 /PRNewswire/ -- In recent years, the rapid development of artificial intelligence has introduced new possibilities across numerous scientific disciplines. As an AI for Science pioneer, DP Technology is continually collaborating with partners to explore the transformative i...
Novel T-cell engager, CDH17 X CD3 cabotamig (ARB202) continues to explore dosing in patients with advanced gastrointestinal cancers
* The second DMC review recommends the continuation of dose escalation in the clinical trial as per protocol. * The interim safety assessment was based on the review of safety data from 18 metastatic gastrointestinal cancer patients, including patients that have tolerated multiple doses of ca...
Fosun Pharma's Self-developed Artemisinin Medicines Inject New Impetus to Malaria Prevention and Treatment in Africa
SHANGHAI, April 26, 2024 /PRNewswire/ -- World Malaria Day is marked each year onApril 25. World Health Organization (WHO) gave as the theme for World Malaria Day 2024Accelerating the fight against malaria for a more equitable world. WHO stated that malaria not only continues to directly endanger...
2024 ASCO | Mabwell to Present Clinical Data of 9MW2821 in Multiple Advanced Solid Tumor
SHANGHAI, April 25, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with an entire industry chain, announced that the Phase I/II clinical study results of the novel Nectin-4-targeting ADC 9MW2821 for multiple advanced solid tumors will be presented in an ...
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 327 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 308 media titles]
2026-02-26 09:30Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 300 media titles]
2026-02-26 19:57