Pharmaceuticals
China Jo-Jo Drugstores Announces $2.74 Million Registered Direct Offering
HANGZHOU, China, May 10, 2024 /PRNewswire/ -- China Jo-Jo Drugstores, Inc. (Nasdaq: CJJD) ("Jo-Jo Drugstores" or the "Company"), a leading online and offline retailer, wholesale distributor of pharmaceutical and other healthcare products and healthcare provider inChina, today announced that it ha...
Kexing Biopharm Facility Passes EU GMP Certification for Paclitaxel for Injection (Albumin Bound)
SHENZHEN, China, May 10, 2024 /PRNewswire/ -- On the evening of May 5, Kexing Biopharm (stock code: 688136) announced it had obtained a "Certificate of GMP Compliance of a Manufacturer" granted by the Norwegian Medical Products Agency in accordance with the European Medicines Agency (EMA) regulat...
Ribobay Pharma boosts CRDMO offerings with Cytiva's first FlexFactory platform for oligonucleotides
* FlexFactory platform for oligonucleotides, an integrated solution to boost efficiency and speed of pharmaceutical manufacturing to meet market demand SHANGHAI, May 10, 2024 /PRNewswire/ -- Ribobay Pharma, a leading Chinese biopharmaceutical contract research, development, and manufacturing o...
WestGene's mRNA Therapeutic Cancer Vaccine Receives FDA Approval
CHENGDU, China, May 10, 2024 /PRNewswire/ -- WestGene, a biotech company dedicated to mRNA technology, announces a historic milestone with the FDA IND approval of its mRNA therapeutic cancer vaccine, WGc-043. This landmark achievement marks the world's first approval of an EB virus-related mRNA ...
SN Bioscience Receives FDA Fast Track Designation for Small Cell Lung Cancer
SEOUL, South Korea, May 9, 2024 /PRNewswire/ -- SN Bioscience Co. Ltd. (CEO Park Young-hwan) announced on May 7 that the FDA has granted Fast Track Designation for small cell lung cancer (SCLC) for SNB-101 (API: SN-38), a new drug for polymer nanoparticle anticancer under clinical trial. SNB-101 ...
Celltrion USA's adalimumab-aaty biosimilar to HUMIRA® now available at low wholesale acquisition cost
* Adalimumab-aaty will be priced at an 85% discount to HUMIRA® (adalimumab) * Branded and unbranded versions of Celltrion USA's adalimumab biosimilar help provide more affordable options for patients JERSEY CITY, N.J., May 9, 2024 /PRNewswire/ -- CelltrionUSA announced today that adalimumab-aa...
FDA Clears the Individualized Nomogram for Rika Plasma Donation System
The latest innovation is anticipated to increase collection volume without increasing collection time * Rika's individualized nomogram, iNomi, can enable plasma donors to donate the right amount of plasma on any given day based on height, weight and hematocrit. * The Rika Plasma Donation Sys...
Infinitopes' Article in Peer-Reviewed Journal Seeks to Unlock the Potential of Cancer Vaccines
Publication in Human Vaccines and Immunotherapeutics highlights progress in understanding tumour biology and vaccine technologies since the pandemic OXFORD, England, May 9, 2024 /PRNewswire/ -- Infinitopes Precision Immunomics, an integrated cancer biotech combining world leading platforms in pr...
Formosa Pharmaceuticals Announces Licensing Agreement with Tabuk Pharmaceuticals, for Commercialization of Clobetasol Propionate Ophthalmic Suspension for the Treatment of Inflammation and Pain Following Ocular Surgery
TAIPEI, May 9, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals ("Formosa", 6838.TWO) announced today that the company has entered into an exclusive licensing agreement with Tabuk Pharmaceuticals Manufacturing Company ("Tabuk"), for exclusive rights to the commercialization of clobetasol...
NeuShen Therapeutics Initiates First-in-Human Trial of NS-136, a Novel Selective M4 Receptor Positive Allosteric Modulator for the Treatment of Schizophrenia
SHANGHAI and LEXINGTON, Mass. , May 8, 2024 /PRNewswire/ -- NeuShen Therapeutics (the "Company"), a clinical-stage global biotechnology company dedicated to pioneering treatments for neurological and psychiatric disorders, today announced the dosing of the first healthy volunteer inAustralia in t...
TiumBio Announces Positive Topline Data from Phase 2a Trial of Merigolix in Patients with Moderate to Severe Endometriosis-Associated Pain
* The study (NCT05138562) met its primary endpoint of change of dysmenorrhea (menstrual pain) score from baseline to 12 weeks compared to placebo across all tested doses (120 mg, 240 mg, and 320 mg) * Merigolix is expected to emerge as a best-in-class treatment based on its excellent pain re...
Minghui Pharmaceutical to Present the Phase I/II Study of MHB088C (B7-H3 ADC) for the Treatment of Patients with Recurrent or Metastatic Solid Tumors in Late-breaking Oral Presentation at the 2024 ASCO Annual Meeting
SHANGHAI, May 8, 2024 /PRNewswire/ -- Minghui Pharmaceutical, Inc., a late-stage clinical biopharmaceutical company focused on autoimmune diseases and oncology, will feature Dr.Lin Shen from Beijing Cancer Hospital at the upcoming ASCO Annual Meeting inChicago. Dr. Shen will present the results f...
FDA Grants Orphan Drug Designation to 9MW2821
SHANGHAI, May 7, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that its self-developed novel Nectin-4-targeting ADC (R&D code: 9MW2821) has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Adminis...
Meihua International Medical Technologies Co., Ltd. to Present at the Emerging Growth Conference on May 9, 2024
Meihua International Medical Technologies Co., Ltd. invites individual and institutional investors as well as advisors and analysts, to attend its real-time, interactive presentation at the Emerging Growth Conference. YANGZHOU, China, May 7, 2024 /PRNewswire/ -- Meihua International Medical Tech...
GC Genome to Present New Clinical Data on Colorectal Cancer Detection at the ASCO Annual Meeting 2024
YONGIN, South Korea, May 7, 2024 /PRNewswire/ -- GC Genome Corporation, a leading diagnostics company, today announced that it will present the new clinical data of its AI-based liquid biopsy platform on colorectal cancer detection at the 2024 American Society of Clinical Oncology (ASCO) Annual ...
HanAll Biopharma Announces Initiation of Phase III Randomized, Double-Masked Vehicle Controlled VELOS-4 Trial Evaluating Tanfanercept for Treatment of Dry Eye Disease
* HanAll has initiated a Phase III VELOS-4 study to evaluate the efficacy and safety of tanfanercept in dry eye based on the findings from the previous Phase III VELOS-3 study. * Tanfanercept demonstrated statistically significant improvement on the secondary outcome measure, Schirmer testing...
Jacobio Pharma Announced its KRAS G12C inhibitor reached the primary endpoint
BEIJING and SHANGHAI and BOSTON, April 30, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced that the data from the Phase II registrational study of the KRAS G12C inhibitor glecirasib were offically reported at the April ASCO Plenary Series, which was held online. Prof. Yuankai Shi, chief ...
Eluminex Biosciences Announces FDA Acceptance of Investigational New Drug (IND) Application for EB-105 - A Novel Trispecific Fusion Antibody for Diabetic Macular Edema (DME) - and Upcoming Scientific Presentations
SAN FRANCISCO and SUZHOU, China, April 30, 2024 /PRNewswire/ -- Eluminex Biosciences (Eluminex), a privately-held biotechnology company focused on the development of advanced protein therapeutics for vision-threatening diseases and dermal facial aesthetics announced the acceptance of their EB-105...
National Medical Products Administration (NMPA) Approves Chipscreen Bioscience's Chidamide (Epidaza) combined with R-CHOP for the treatment of diffuse large B-cell lymphoma
SHENZHEN, China, April 30, 2024 /PRNewswire/ -- Shenzhen Chipscreen Biosciences Co., Ltd. (Chipscreen Biosciences, Stock Symbol: 688321.SH) announced that the company's lead innovative product Chidamide (Epidaza®) , an oral subtype-selective histone deacetylase (HDAC) inhibitor, combined with R-C...
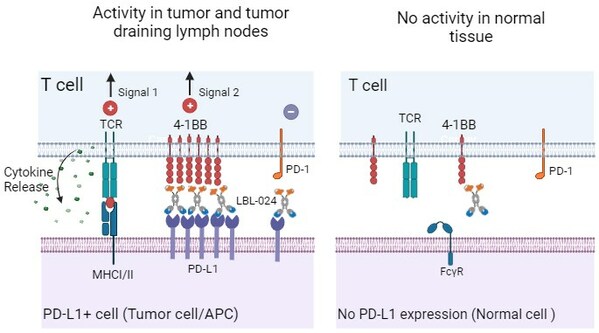
A Potential First-In-Class Drug: CDE Approved Single-Arm Pivotal Clinical Study of LBL-024, An Anti-PD-L1/4-1BB Bispecific Antibody Developed by Leads Biolabs
NANJING, China, April 30, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. (hereinafter referred to as "Leads Biolabs") announced that LBL-024, an anti-PD-L1/4-1BB bispecific antibody independently developed by Leads Biolabs with global intellectual property rights has received approval to co...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 320 media titles]
2026-02-02 10:00Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 320 media titles]
2026-01-29 15:06Agoda Launches Open-Source API Agent to Simplify MCP Server Integrations
[Picked up by 311 media titles]
2026-01-27 13:00Nokia Strengthens Edge AI Capabilities Through Strategic Collaboration with Blaize on Hybrid Inference Solutions Across Asia Pacific Regions
[Picked up by 294 media titles]
2026-01-27 18:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 286 media titles]
2026-02-01 19:35