Pharmaceuticals
Innovent Releases Final Analysis Results of ORIENT-16: the Phase 3 Study of Sintilimab in Combination with Chemotherapy for the First-Line Treatment of Gastric Cancer at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 17, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
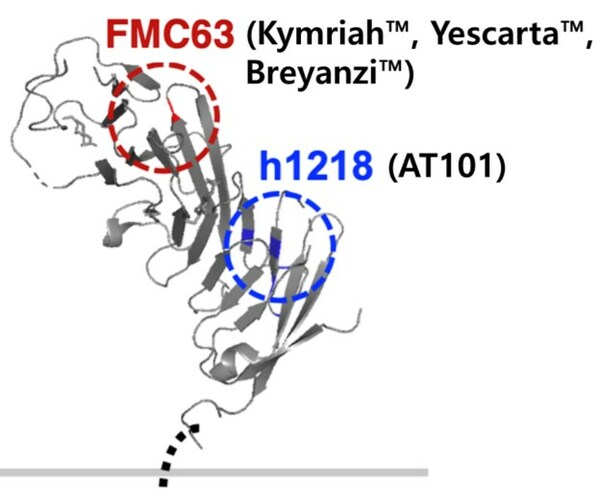
Promising Efficacy of AT101 CAR-T for Blood Cancer
SEOUL, South Korea, April 17, 2023 /PRNewswire/ -- AbClon, a South Korean biotech firm, presented non-clinical and phase 1 interim results of its AT101 novel CAR-T therapy at the annual 2023 AACR conference. AT101 targets the CD19 protein for treatment of blood cancer. AT101 demonstrated superio...
Asieris Releases 2022 Annual Report: Makes Steady Progress in its Clinical Development, Global Expansion to Treat Genitourinary Tumors, and Implementing its Commercialization Strategy to Provide Integrated Diagnosis-to-Treatment Solutions
SHANGHAI, April 17, 2023 /PRNewswire/ -- Asieris Pharmaceuticals(688176.SH), a global biopharma company specializing in the discovery, development and commercialization of innovative drugs that treat genitourinary (GU) tumors and other related diseases, today released its 2022 annual report. Whil...
Bassem Elmankabadi, M.D. joins Foresee Pharmaceuticals as a Senior Vice President of Clinical Development
TAIPEI, April 17, 2023 /PRNewswire/ -- Foresee Pharmaceuticals Co., Ltd. (6576.TWO) ("Foresee"), announces today thatBassem Elmankabadi, M.D. joins Foresee as a Senior Vice President of Clinical Development. Dr. Bassem Elmankabadi is a Board Certified Surgeon with over 17 years of clinical backg...
Innovent Releases Final Analysis Results of ORIENT-15: the Phase 3 Study of Sintilimab plus Chemotherapy for the First-Line Treatment of Esophageal Squamous Cell Carcinoma at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 16, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
Minghui Pharmaceutical Inc. Announces Phase 2 Clinical Trial Success of MH004 Cream as a Potential Best-in-Class Topical Treatment for Mild-to-Moderate Atopic Dermatitis and the FDA Clearance of its Global Phase 3 MRCT
* Study met both primary and all key secondary endpoints * Mean percentage change from baseline in Eczema Area and Severity Index (EASI) score was -78.7% in 1.0% MH004 Cream-treated group compared to -46.7% in vehicle group * 79.6% individuals treated with 1.0% MH004 Cream achieved EASI-75 c...
Zenshine Pharmaceuticals' ZX-7101A Met the Primary Endpoint of the Phase 2 Study for the Treatment of Acute Uncomplicated Influenza in Adults
NANJING, China, April 13, 2023 /PRNewswire/ -- On April 1st, 2023, during the First Infection Collaboration Forum of the Chinese National Center for Infectious Diseases, Nanjing Zenshine Pharmaceuticals Co., Ltd. (Zenshine Pharmaceuticals) announced that its proprietary anti-influenza agent ZX-71...
Viva Biotech Attended SAPA-China
HONG KONG, April 12, 2023 /PRNewswire/ -- 6th-7th April, the "2022 SAPA-China Annual Conference - Opportunities and Challenges for the Development ofChina's Biopharmaceutical Industry" hosted by The Sino-American Pharmaceutical Professionals Association (SAPA) was held inChengdu, Sichuan. Viva Bi...
Bridge Biotherapeutics Announces First Patient Dosed in its Phase 2a Clinical Trial of BBT-877, a Potent Autotaxin Inhibitor for the Treatment of Idiopathic Pulmonary Fibrosis
SEONGNAM, South Korea and CAMBRIDGE, Mass., April 12, 2023 /PRNewswire/ -- Bridge Biotherapeutics (KQ288330), a clinical-stage biotech company based in South Korea developing novel drugs for fibrosis, cancer, and inflammation, announced it has dosed the first patient in its Phase 2a clinical study...
Standigm announces Younsung Choo as chief executive officer
Former LG life Sciences exec and long-time Standigm advisor brings extensive
experience in pharmaceuticals and project management
SEOUL, South Korea, April 12, 2023 /PRNewswire/ -- Standigm
GC Genome-KAIST team Announces Publication of a Groundbreaking AI-Based Liquid Biopsy Technology for Multi-Cancer Early Detection and Localization in Nature Communications
YONGIN, South Korea, April 11, 2023 /PRNewswire/ -- GC Genome Corporation, a leading genomic diagnostics company, today announced the publication of a new study inNature Communications, showcasing the company's novel AI-based liquid biopsy technology. The study highlights the unprecedented accura...
Specialised Therapeutics signs partnership agreement with Akeso Inc. and CTTQ-Akeso to commercialise new anti-PD1 antibody in Australia and Southeast Asia
SINGAPORE, April 11, 2023 /PRNewswire/ -- Independent biopharmaceutical company Specialised Therapeutics Asia Pte Ltd (ST) will partner with CTTQ-Akeso ( Shanghai) Biomed. Tech. Co., Ltd. (CTTQ-Akeso)(jointly established by Akeso, Inc. (9926.HK, Akeso) and Chia Tai Tianqing Pharmaceutical Group Co...
OnCusp Therapeutics Showcases Potent Anti-Tumor Activity of its CDH6 ADC at AACR Annual Meeting 2023
NEW YORK, April 11, 2023 /PRNewswire/ -- OnCusp Therapeutics, a global biopharmaceutical company dedicated to transforming cutting-edge preclinical innovation into clinically validated treatments for cancer patients worldwide, today announced that the preclinical data on its lead program CUSP0...
Asieris' subsidiary makes significant progress in its commercialization strategy by obtaining the "Drug Distribution License"
SHANGHAI, April 10, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced that its subsidiary, Hainan Yahong ph...
China's First Nectin-4 Targeted ADC 9MW2821 Clinical Progress Released
SHANGHAI, April 6, 2023 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with the whole industry chain layout, announced its Nectin-4 targeted site-specific ADC asset (R&D code: 9MW2821) demonstrated promising clinical data. The preliminary data show positive therapeut...
Nuance Pharma Announces Dosing of First Patient in ENHANCE - China Phase III Trial for Chronic Obstructive Pulmonary Disease ("COPD")
SHANGHAI, April 6, 2023 /PRNewswire/ -- Nuance Pharma ("the Company") announces dosing of first patient in the ENHANCE - China Phase III Trial for the maintenance treatment of chronic obstructive pulmonary disease ("COPD") of its novel solution Ensifentrine in mainlandChina. Ensifentrine is a fi...
BIORCHESTRA Expands Executive Leadership Team with the Appointment of David Oxley as Chief Business Officer
CAMBRIDGE, Mass. and DAEJEON, South Korea, April 5, 2023 /PRNewswire/ --
BIORCHESTRA
Biosion Initiates Phase II Clinical Trial of BSI-045B in Atopic Dermatitis
NEWARK, Del. and NANJING, China, April 4, 2023 /PRNewswire/ -- Biosion USA, Inc. (Biosion), a global clinical-stage R&D biotechnology company, today announced that it has successfully opened an IND following review by the U.S. Food and Drug Administration toinitiate a Phase 2 clinical trial of BS...
Lynk Pharmaceuticals Announces Positive Results for LNK01003 in Phase I Trial in Healthy Subjects
HANGZHOU, China, April 4, 2023 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, announced that it has recently completed phase I trials in healthy subjects inChina for its self-developed innovative intestina...
GC Cell and New Management Embarking on a New Chapter
* Securing future growth engine by scouting global talents; AGM resolved new CEOJames Park and CTO Ho-won Kim as internal directors * Setting business goals focusing on change and growth * Proposing a new corporate vision of "Global Creator of Cell & Gene Therapy" YONGIN,South Korea, April 3...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 309 media titles]
2026-02-03 11:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 302 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 286 media titles]
2026-02-01 19:35