Pharmaceuticals
PharmaBlock Opens New R&D Facility in Pennsylvania -- Expanding Capacity to Deliver GMP Projects
WEST CHESTER, Pa., March 27, 2023 /PRNewswire/ -- PharmaBlock (stock code: 300725.SZSE), a global, fully integrated CDMO company focused on innovative chemistry and low-carbon manufacturing, announces the opening of its new R&D facility inWest Chester, Pennsylvania. The facility expands the comp...
111, Inc. Announces Fourth Quarter and Fiscal Year 2022 Financial Results
SHANGHAI, March 23, 2023 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced its unaudited financial results for the fourth qua...
Ascentage Pharma Announces 2022 Annual Results Including Strong Sales of Olverembatinib and Steady Progress in Transition Towards Biopharma
SUZHOU, China and ROCKVILLE, Md., March 22, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced its annual results for the full year 2022. During...
Jacobio Pharma Announces 2022 Annual Results
BEIJING, SHANGHAI and BOSTON, March 22, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced its 2022 annual results. R&D investment wasRMB530 million, showing an increase of 26% compared with 2021. The revenue wasRMB95.7 million, which was generated from an out-licensing deal. Jacobio Pharma ...
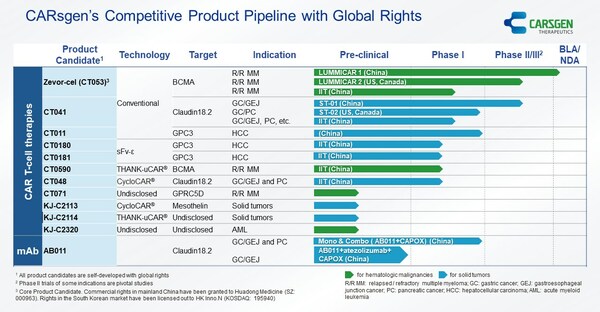
CARsgen Announced 2022 Annual Results and Business Updates
SHANGHAI, March 22, 2023 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announced its 2022 Annual Results. Business Highlights * Zevor-cel (CT053)...
Biosion bispecific antibody therapies to be featured at the American Association for Cancer Research (AACR) annual meeting 2023
NEWARK, Del., March 22, 2023 /PRNewswire/ -- Biosion USA, Inc. (Biosion), a global R&D biotechnology company, today announced the upcoming presentations of non-clinical data from its oncology pipeline, including BSI-507: anti-PD1ⅹanti-PVRIG bispecific antibody and BSI-508: anti-PD1ⅹanti-CD47 bis...
WuXi Biologics Reports Remarkable 2022 Annual Results
Revenue Increased by 48.4% Y-o-Y to RMB15,268.7 Million Gross Profit Increased by 39.2% Y-o-Y to RMB6,724.0 Million Adjusted Net Profit Rose by 47.1% to RMB5,053.9 Million Non-COVID Revenue Achieved 63% Y-o-Y Growth, Strong Momentum Continues into 2023 and Beyond "R" in CRDMO Business Model A...
Jacobio announces clinical collaboration to evaluate CD73 monoclonal antibody JAB-BX102 in combination with KEYTRUDA® (pembrolizumab) for patients with cancer
BEIJING, SHANGHAI and BOSTON, March 22, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced it has entered into a clinical collaboration with Merck & Co., Inc.,Rahway, NJ, USA to evaluate the combination of Jacobio's CD73 monoclonal antibody JAB-BX102 in combination with Merck & Co., Inc., Ra...
280Bio Provides Update on the KRAS Inhibitor TEB-17231 at the 2023 AACR Annual Meeting
A broadly acting KRAS Inhibitor, TEB-17231, robustly blocks tumor growth and overcomes KRASG12C inhibitor mediated resistance SHANGHAI, March 21, 2023 /PRNewswire/ -- 280Bio, Inc. a clinical stage biotechnology company, focused on the development of precision oncology medicines, today announced ...
Kintor Pharma's KX-826 and GT20029 for Treatment of Androgenetic Alopecia (AGA) and Acne Presented at AAD 2023
SUZHOU, China, March 21, 2023 /PRNewswire/ -- The 2023 Annual Meeting of American Academy of Dermatology Association (AAD 2023) was held from 17 to21 March 2023 in New Orleans, Louisiana, United States. As one of the largest, most influential and representative of all dermatologic associations, ...
Hovione and Ripple Enter Strategic Partnership to Expand Epidel® Platform into Non-Ophthalmic Space
LISBON, Portugal, March 20, 2023 /PRNewswire/ -- Hovione, the specialist integrated CDMO, leader in spray drying and particle engineering, and Ripple Therapeutics, a leading ophthalmic sustained drug delivery company, have entered a strategic partnership to expand the use of Ripple's Epidel® plat...
CNEY Wins a Large Activated Carbon Order
LISHUI, China, March 20, 2023 /PRNewswire/ -- CN Energy Group. Inc. (NASDAQ: CNEY) ("CNEY", or the "Company") today announced that its wholly-owned subsidiary, Zhejiang CN Energy New Materials Co., Ltd., has recently signed a contract to supply its high-quality wood-based activated carbon to a bu...
I-Mab to Report Full Year 2022 Financial Results and Provide Corporate Update on March 31, 2023
GAITHERSBURG, Md. and SHANGHAI, China, March 20, 2023 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, today announced that the Company will report financial results ...
Hanmi Pharmaceutical's Combination Drug for Dyslipidemia Treatment, 'Rosuzet': Confirmed Non-inferiority Efficacy and Safety Compared to Monotherapy in 'The Lancet' & 'European Heart Journal'
SEOUL, South Korea, March 17, 2023 /PRNewswire/ -- Hanmi Pharmaceuticals (128940.KS), a subsidiary of Hanmi Science (008930.KS) in Korea, announced that the results of large-scale clinical trial with Rosuzet have been published in 'The Lancet' and the 'European Heart Journal'. R&D-oriented pha...
Samsung Biologics announces construction of Plant 5 to commence Bio Campus II capacity expansion
* The company plans to begin construction in the first half of 2023 and commence operations in 2025 * As part of Samsung Biologics' second Bio Campus, Plant 5 will hold a manufacturing capacity of 180,000 liters, expanding the company's total site capacity to 784,000 liters upon its completio...
Nav1.7 Selective Inhibitor OLP-1002 Shows Strong Efficacy and Long Therapeutic Duration according to Interim Findings from a Phase 2a Study in OA Patients
SEOUL, South Korea, March 16, 2023 /PRNewswire/ -- OliPass Corporation, an RNA therapeutics platform company, disclosed that OLP-1002, a selective inhibitor of Nav1.7 sodium ion channel, showed strong analgesic efficacy and long therapeutic duration according to interim findings from a placebo-co...
FDA Approves Expanded Indication for Telix's Illuccix® to Include Patient Selection for PSMA-Directed Radioligand Therapy
INDIANAPOLIS, March 16, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that the United States Food and Drug Administration (FDA) has approved a supplementary New Drug Application (sNDA) for Illuccix® (kit for the preparation of gallium Ga 68 goze...
Akeso Reported 2022 Annual Results
* Strong sales fully demonstrated Akeso's commercialization ability: Products sales totaledRMB1,104.4 million, increasing 422% .开坦尼® (cadonilimab) recorded strong sales ofRMB546.3 million in its first six months of approval in China. 安尼可®(penpulimab injection, PD-1) recorded product sales ofRMB...
Bridge Biotherapeutics to Present Updated Preclinical Data of BBT-207 at the AACR 2023 Annual Meeting
* The abstract of BBT-207 preclinical studies is now available on the AACR
website
HanAll Biopharma Opens Applications for the 2023 Pharmaceutical Industry Fellowship Program
* Applications for physicians trained in Indonesia now open until April 15, 2023 12:00 AM * The Pharmaceutical Industry Fellowship Program provides physicians with the opportunity to experience the various steps of the drug development process ROCKVILLE, Md. and SEOUL, South Korea, March 15, ...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 304 media titles]
2026-02-03 11:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 300 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 286 media titles]
2026-02-01 19:35