Pharmaceuticals
BIORCHESTRA Announces Significant Progress in Studies of BMD-001 Drug Program
* Encouraging non-human primate (NHP) findings support the progression of IV-formulatedBMD-001 ahead of formal clinical studies for Alzheimer's disease, Amyotrophic Lateral Sclerosis, and Parkinson's disease * Further validation of Brain Targeting RNAi Nanomedicine (BTRiNTM), including target...
Four innovative drugs launched in three years, HKEX -Listed Simcere Pharmaceutical Group is on the Fast Track Toward Building an Innovation-Driven Global Pharmaceutical Company
-The revenue from innovative drugs accounted for more than 65% of total revenue -Simcere's total revenue and the revenue from innovative drugs hit record highs. NANJING, China, April 24, 2023 /PRNewswire/ -- On Apr. 18, Simcere Pharmaceutical (2096.HK) disclosed the latest progress on the compa...
Tibetan medicine advanced over past decade
BEIJING, April 24, 2023 /PRNewswire/ -- A news report from chinadaily.com.cn: Adhering to an equal emphasis on traditional Chinese and Western medicine, the government of the Tibet autonomous region has increased financial investment, formulated supportive policies and promoted the development of...
Samsung Biologics Reports First Quarter 2023 Financial Results
* Recorded Q1'23 consolidated revenue of KRW 720.9 billion. * Recorded Q1'23 consolidated operating profit of KRW 191.7 billion. * Expected to maintain a strong growth trajectory with the full utilization of existing and newly expanded capacities. INCHEON, South Korea, April 24, 2023 /PRNews...
Patsnap Unveils Synapse Visitor, the Latest AI-Powered Pharmaceutical Intelligence Platform
LONDON, April 23, 2023 /PRNewswire/ -- On April 20, Patsnap, the world's leading SaaS provider for intelligence, announced the launch of its latest version of the intelligence platform, Synapse, available to visitors. This version includes advanced search functions for drugs and pharmaceutical o...
ImmVira Appoints Dr. Oliver Kong as Chief Medical Officer
SHENZHEN, China, April 20, 2023 /PRNewswire/ -- On April 17, 2023, ImmVira announced the appointment of Dr.Oliver Kong as Chief Medical Officer (CMO). Dr. Kong will be fully in charge of global clinical development and international collaboration of company pipelines, and participate in overall c...
CARsgen's CAR T-cell Candidate CT041 Achieves IND Clearance from the NMPA for the Postoperative Adjuvant Therapy of Pancreatic Cancer
SHANGHAI, April 20, 2023 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announced that CT041, an autologous CAR T-cell product candidate against the p...
Samyang Holdings Enters Partnership with LG Chem for the Development of Innovative Cancer Therapeutics
* Implementation of Samyang Holdings' drug delivery technology for the development of mRNA-based cancer therapeutics SEOUL, South Korea, April 20, 2023 /PRNewswire/ -- Samyang Holdings and LG Chem have announced their recent execution of strategic partnership agreement onThursday, April 20th, f...
Innovent Announces Overall Survival Results of Phase 2 Study of Pemazyre® (pemigatinib) in Chinese Patients with Advanced Cholangiocarcinoma Presented at AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 19, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
500 women join Australia's landmark maternity medical device study
SYDNEY, April 19, 2023 /PRNewswire/ -- Medical device company Baymatob has successfully completed one ofAustralia's largest maternity health studies for a medical device, enrolling 500 mothers in a pilot study to evaluate its AI-guided labor monitoring device, Oli. The device is the only product ...
Crystal Pharmatech's CDMO Business Unit - Crystal Formulation Services Received China Drug Product Manufacturing License, Achieving the Important Milestone in Formulation Capability
SUZHOU, China, April 19, 2023 /PRNewswire/ -- Crystal Formulation Services, a subsidiary of Crystal Pharmatech, has achieved an exciting new milestone in its formulation capabilities. The company was recently granted the "Drug Manufacturing License" (C Certificate) by the Jiangsu Medical Products...
China Jo-Jo Drugstores Announces the Termination of the Previously Proposed Registered Direct Offering
HANGZHOU, China, April 19, 2023 /PRNewswire/ -- China Jo-Jo Drugstores, Inc. (Nasdaq: CJJD) ("Jo-Jo Drugstores" or the "Company"), a leading online and offline retailer, wholesale distributor of pharmaceutical and other healthcare products and healthcare provider inChina, announced today that the...
Cali Biosciences Initiates Phase III Studies of CPL-01 (Long-Acting Ropivacaine) to Decrease Post-Operative Pain and Reduce or Eliminate Opioid Use
- Subjects in Herniorrhaphy Study Dosed - Successful FDA Interactions on Two Pre-clinical Pipeline Products Also Discussed SAN DIEGO, April 18, 2023 /PRNewswire/ -- Cali Biosciences Co., Ltd. (hereinafter referred to as "Cali" or "Cali Biosciences"), a biopharmaceutical company focused on the re...
AACR 2023 | ImmVira presented preclinical study results of first CAR-T enabler oncolytic product MVR-T7011 at AACR Annual Meeting
SHENZHEN, China, April 17, 2023 /PRNewswire/ -- On April 18, 2023, U.S. time,
ImmVira presented preclinical study results of CAR-T enabler oHSV product
MVR-7011 through poster publication at the American Association for Cancer
Research ("AACR") annual meeting.
Bridge Biotherapeutics Releases Updated Preclinical Data for BBT-207 at the AACR Annual Meeting
* New preclinical data validate anti-tumor efficacy and intracranial activity of the drug candidate * Bridge submitted an IND application to the U.S. Food and Drug Administration in March, and expects to initiate the first-in-human study of BBT-207 this year SEONGNAM, South Korea and CAMBRIDG...
Technoderma Medicines Initiates TDM-105795 Androgenetic Alopecia Phase 2 Clinical Trial
CHENGDU, China, April 18, 2023 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report that the Company has begun dosing patients in its Androgenetic Alopecia (AGA) Phase 2 clinical trial (NCT05802173) of topical TDM-105795 sol...
GC Genome to Present the Brand New Liquid Biopsy Platform for Cancer Detection at AACR Annual Meeting 2023
YONGIN, South Korea, April 18, 2023 /PRNewswire/ -- GC Genome Corporation, a leading diagnostics company, today announced that the company will present data showcasing the deep learning algorithm that detects and classifies multi-cancer using methylation information and genomic data at the Americ...
GC Biopharma celebrates 'World Hemophilia Day'
* To display a large media façade on its Yong-in R&D Center YONGIN, South Korea, April 17, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a leading provider of biopharmaceutical products inSouth Korea, takes part in a campaign to celebrate World Hemophilia Day. The company announced that a l...
Innovent Releases Final Analysis Results of ORIENT-16: the Phase 3 Study of Sintilimab in Combination with Chemotherapy for the First-Line Treatment of Gastric Cancer at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, April 17, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology a...
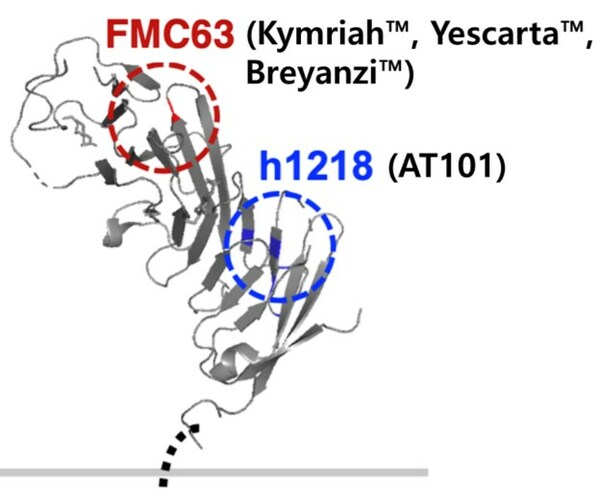
Promising Efficacy of AT101 CAR-T for Blood Cancer
SEOUL, South Korea, April 17, 2023 /PRNewswire/ -- AbClon, a South Korean biotech firm, presented non-clinical and phase 1 interim results of its AT101 novel CAR-T therapy at the annual 2023 AACR conference. AT101 targets the CD19 protein for treatment of blood cancer. AT101 demonstrated superio...
Week's Top Stories
Most Reposted
HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 310 media titles]
2026-02-26 09:30Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 308 media titles]
2026-02-26 19:57Visa partners with Laufey to spread the magic of travel in Asia Pacific; to be Official Payment Partner for Laufey: A Matter of Time Tour
[Picked up by 303 media titles]
2026-03-04 12:35SMU and Fudan Launch Region's First Tech-Focused DBA
[Picked up by 300 media titles]
2026-03-02 09:15Infobip is set to launch AgentOS to orchestrate autonomous AI-driven customer journeys at scale
[Picked up by 289 media titles]
2026-03-02 09:00